Blood-stage Plasmodium infection induces CD8+ T lymphocytes to parasite-expressed antigens, largely regulated by CD8alpha+ dendritic cells
- PMID: 18799734
- PMCID: PMC2567226
- DOI: 10.1073/pnas.0806727105
Blood-stage Plasmodium infection induces CD8+ T lymphocytes to parasite-expressed antigens, largely regulated by CD8alpha+ dendritic cells
Abstract
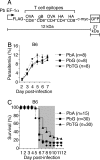
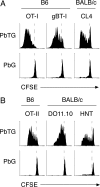
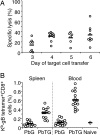
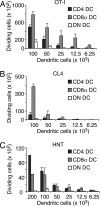
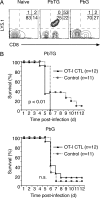
Although CD8(+) T cells do not contribute to protection against the blood stage of Plasmodium infection, there is mounting evidence that they are principal mediators of murine experimental cerebral malaria (ECM). At present, there is no direct evidence that the CD8(+) T cells mediating ECM are parasite-specific or, for that matter, whether parasite-specific CD8(+) T cells are generated in response to blood-stage infection. To resolve this and to define the cellular requirements for such priming, we generated transgenic P. berghei parasites expressing model T cell epitopes. This approach was necessary as MHC class I-restricted antigens to blood-stage infection have not been defined. Here, we show that blood-stage infection leads to parasite-specific CD8(+) and CD4(+) T cell responses. Furthermore, we show that P. berghei-expressed antigens are cross-presented by the CD8alpha(+) subset of dendritic cells (DC), and that this induces pathogen-specific cytotoxic T lymphocytes (CTL) capable of lysing cells presenting antigens expressed by blood-stage parasites. Finally, using three different experimental approaches, we provide evidence that CTL specific for parasite-expressed antigens contribute to ECM.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Development of a Novel CD4+ TCR Transgenic Line That Reveals a Dominant Role for CD8+ Dendritic Cells and CD40 Signaling in the Generation of Helper and CTL Responses to Blood-Stage Malaria.J Immunol. 2017 Dec 15;199(12):4165-4179. doi: 10.4049/jimmunol.1700186. Epub 2017 Oct 30. J Immunol. 2017. PMID: 29084838 Free PMC article.
-
IL-4Rα signaling by CD8α+ dendritic cells contributes to cerebral malaria by enhancing inflammatory, Th1, and cytotoxic CD8+ T cell responses.J Biol Chem. 2021 Jan-Jun;296:100615. doi: 10.1016/j.jbc.2021.100615. Epub 2021 Mar 30. J Biol Chem. 2021. PMID: 33798555 Free PMC article.
-
Eosinophils Suppress the Migration of T Cells Into the Brain of Plasmodium berghei-Infected Ifnar1-/- Mice and Protect Them From Experimental Cerebral Malaria.Front Immunol. 2021 Sep 30;12:711876. doi: 10.3389/fimmu.2021.711876. eCollection 2021. Front Immunol. 2021. PMID: 34659202 Free PMC article.
-
Pathogenic CD8+ T cells in experimental cerebral malaria.Semin Immunopathol. 2015 May;37(3):221-31. doi: 10.1007/s00281-015-0476-6. Epub 2015 Mar 13. Semin Immunopathol. 2015. PMID: 25772948 Review.
-
Dendritic cell function and antigen presentation in malaria.Curr Opin Immunol. 2016 Jun;40:1-6. doi: 10.1016/j.coi.2016.01.010. Epub 2016 Feb 1. Curr Opin Immunol. 2016. PMID: 26845735 Review.
Cited by
-
Nitric oxide is involved in the upregulation of IFN-γ and IL-10 mRNA expression by CD8⁺ T cells during the blood stages of P. chabaudi AS infection in CBA/Ca mice.Int J Biol Sci. 2011;7(9):1401-11. doi: 10.7150/ijbs.7.1401. Epub 2011 Nov 1. Int J Biol Sci. 2011. PMID: 22110391 Free PMC article.
-
Brain microvessel cross-presentation is a hallmark of experimental cerebral malaria.EMBO Mol Med. 2013 Jul;5(7):984-99. doi: 10.1002/emmm.201202273. Epub 2013 May 16. EMBO Mol Med. 2013. PMID: 23681698 Free PMC article.
-
Phenotypic and functional profiling of malaria-induced CD8 and CD4 T cells during blood-stage infection with Plasmodium yoelii.Immunology. 2011 Feb;132(2):273-86. doi: 10.1111/j.1365-2567.2010.03363.x. Epub 2010 Oct 29. Immunology. 2011. PMID: 21039472 Free PMC article.
-
Erythropoietin protects against murine cerebral malaria through actions on host cellular immunity.Infect Immun. 2014 Jan;82(1):165-73. doi: 10.1128/IAI.00929-13. Epub 2013 Oct 14. Infect Immun. 2014. PMID: 24126529 Free PMC article.
-
The subcellular location of ovalbumin in Plasmodium berghei blood stages influences the magnitude of T-cell responses.Infect Immun. 2014 Nov;82(11):4654-65. doi: 10.1128/IAI.01940-14. Epub 2014 Aug 25. Infect Immun. 2014. PMID: 25156724 Free PMC article.
References
-
- Good MF, Doolan DL. Immune effector mechanisms in malaria. Curr Opin Immunol. 1999;11:412–419. - PubMed
-
- Renia L, et al. Pathogenic T cells in cerebral malaria. Int J Parasitol. 2006;36:547–554. - PubMed
-
- Belnoue E, et al. On the pathogenic role of brain-sequestered αβ CD8+ T cells in experimental cerebral malaria. J Immunol. 2002;169:6369–6375. - PubMed
-
- Hermsen C, van de Wiel T, Mommers E, Sauerwein R, Eling W. Depletion of CD4+ or CD8+ T cells prevents Plasmodium berghei induced cerebral malaria in end-stage disease. Parasitology. 1997;114:7–12. - PubMed
-
- Yanez DM, Manning DD, Cooley AJ, Weidanz WP, van der Heyde HC. Participation of lymphocyte subpopulations in the pathogenesis of experimental murine cerebral malaria. J Immunol. 1996;157:1620–1624. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

