The TRIM5alpha B-box 2 domain promotes cooperative binding to the retroviral capsid by mediating higher-order self-association
- PMID: 18799578
- PMCID: PMC2583650
- DOI: 10.1128/JVI.01548-08
The TRIM5alpha B-box 2 domain promotes cooperative binding to the retroviral capsid by mediating higher-order self-association
Abstract
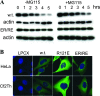
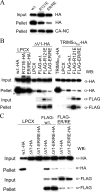
The retroviral restriction factor, TRIM5alpha, blocks infection of a spectrum of retroviruses soon after virus entry into the cell. TRIM5alpha consists of RING, B-box 2, coiled-coil, and B30.2(SPRY) domains. The B-box 2 domain is essential for retrovirus restriction by TRIM5alpha, but its specific function is unknown. We show here that the B-box 2 domain mediates higher-order self-association of TRIM5alpha(rh) oligomers. This self-association increases the efficiency of TRIM5alpha binding to the retroviral capsid, thus potentiating restriction of retroviral infection. The contribution of the B-box 2 domain to cooperative TRIM5alpha association with the retroviral capsid explains the conditional nature of the restriction phenotype exhibited by some B-box 2 TRIM5alpha mutants; the potentiation of capsid binding that results from B-box 2-mediated self-association is essential for restriction when B30.2(SPRY) domain-mediated interactions with the retroviral capsid are weak. Thus, B-box 2-dependent higher-order self-association and B30.2(SPRY)-dependent capsid binding represent complementary mechanisms whereby sufficiently dense arrays of capsid-bound TRIM5alpha proteins can be achieved.
Figures

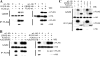

Similar articles
-
Virus-specific effects of TRIM5α(rh) RING domain functions on restriction of retroviruses.J Virol. 2013 Jul;87(13):7234-45. doi: 10.1128/JVI.00620-13. Epub 2013 May 1. J Virol. 2013. PMID: 23637418 Free PMC article.
-
Functional interplay between the B-box 2 and the B30.2(SPRY) domains of TRIM5alpha.Virology. 2007 Sep 30;366(2):234-44. doi: 10.1016/j.virol.2007.04.022. Epub 2007 May 31. Virology. 2007. PMID: 17543365 Free PMC article.
-
A B-box 2 surface patch important for TRIM5alpha self-association, capsid binding avidity, and retrovirus restriction.J Virol. 2009 Oct;83(20):10737-51. doi: 10.1128/JVI.01307-09. Epub 2009 Aug 5. J Virol. 2009. PMID: 19656869 Free PMC article.
-
Retroviral restriction factors TRIM5α: therapeutic strategy to inhibit HIV-1 replication.Curr Med Chem. 2011;18(17):2649-54. doi: 10.2174/092986711795933687. Curr Med Chem. 2011. PMID: 21568899 Review.
-
TRIM5alpha.Curr Top Microbiol Immunol. 2009;339:47-66. doi: 10.1007/978-3-642-02175-6_3. Curr Top Microbiol Immunol. 2009. PMID: 20012523 Review.
Cited by
-
Hexagonal assembly of a restricting TRIM5alpha protein.Proc Natl Acad Sci U S A. 2011 Jan 11;108(2):534-9. doi: 10.1073/pnas.1013426108. Epub 2010 Dec 27. Proc Natl Acad Sci U S A. 2011. PMID: 21187419 Free PMC article.
-
Primate TRIM34 is a broadly-acting, TRIM5-dependent lentiviral restriction factor.bioRxiv [Preprint]. 2023 Mar 25:2023.03.24.534139. doi: 10.1101/2023.03.24.534139. bioRxiv. 2023. Update in: Retrovirology. 2023 Aug 22;20(1):15. doi: 10.1186/s12977-023-00629-4 PMID: 36993223 Free PMC article. Updated. Preprint.
-
The Three-Fold Axis of the HIV-1 Capsid Lattice Is the Species-Specific Binding Interface for TRIM5α.J Virol. 2018 Feb 12;92(5):e01541-17. doi: 10.1128/JVI.01541-17. Print 2018 Mar 1. J Virol. 2018. PMID: 29237846 Free PMC article.
-
Virus-specific effects of TRIM5α(rh) RING domain functions on restriction of retroviruses.J Virol. 2013 Jul;87(13):7234-45. doi: 10.1128/JVI.00620-13. Epub 2013 May 1. J Virol. 2013. PMID: 23637418 Free PMC article.
-
Adaptation of HIV-1 to cells expressing rhesus monkey TRIM5α.Virology. 2010 Dec 20;408(2):204-12. doi: 10.1016/j.virol.2010.09.019. Epub 2010 Oct 16. Virology. 2010. PMID: 20956011 Free PMC article.
References
-
- Cao, T., K. L. Borden, P. S. Freemont, and L. D. Etkin. 1997. Involvement of the rfp tripartite motif in protein-protein interactions and subcellular distribution. J. Cell Sci. 110(Pt. 14)1563-1571. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

