Human squamous cell carcinomas evade the immune response by down-regulation of vascular E-selectin and recruitment of regulatory T cells
- PMID: 18794336
- PMCID: PMC2556796
- DOI: 10.1084/jem.20071190
Human squamous cell carcinomas evade the immune response by down-regulation of vascular E-selectin and recruitment of regulatory T cells
Abstract
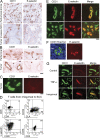
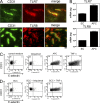
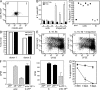
Squamous cell carcinomas (SCCs) of the skin are sun-induced skin cancers that are particularly numerous in patients on T cell immunosuppression. We found that blood vessels in SCCs did not express E-selectin, and tumors contained few cutaneous lymphocyte antigen (CLA)(+) T cells, the cell type thought to provide cutaneous immunosurveillance. Tumors treated with the Toll-like receptor (TLR)7 agonist imiquimod before excision showed induction of E-selectin on tumor vessels, recruitment of CLA(+) CD8(+) T cells, and histological evidence of tumor regression. SCCs treated in vitro with imiquimod also expressed vascular E-selectin. Approximately 50% of the T cells infiltrating untreated SCCs were FOXP3(+) regulatory T (T reg) cells. Imiquimod-treated tumors contained a decreased percentage of T reg cells, and these cells produced less FOXP3, interleukin (IL)-10, and transforming growth factor (TGF)-beta. Treatment of T reg cells in vitro with imiquimod inhibited their suppressive activity and reduced FOXP3, CD39, CD73, IL-10, and TGF-beta by indirect mechanisms. In vivo and in vitro treatment with imiquimod also induced IL-6 production by effector T cells. In summary, we find that SCCs evade the immune response at least in part by down-regulating vascular E-selectin and recruiting T reg cells. TLR7 agonists neutralized both of these strategies, supporting their use in SCCs and other tumors with similar immune defects.
Figures




Similar articles
-
Nitric oxide-producing myeloid-derived suppressor cells inhibit vascular E-selectin expression in human squamous cell carcinomas.J Invest Dermatol. 2012 Nov;132(11):2642-51. doi: 10.1038/jid.2012.190. Epub 2012 Jun 21. J Invest Dermatol. 2012. PMID: 22718118 Free PMC article.
-
Imiquimod enhances IFN-gamma production and effector function of T cells infiltrating human squamous cell carcinomas of the skin.J Invest Dermatol. 2009 Nov;129(11):2676-85. doi: 10.1038/jid.2009.151. Epub 2009 Jun 11. J Invest Dermatol. 2009. PMID: 19516264 Free PMC article.
-
Signaling through TLR7 enhances the immunosuppressive activity of murine CD4+CD25+ T regulatory cells.J Leukoc Biol. 2010 Jan;87(1):117-25. doi: 10.1189/jlb.0908559. Epub 2009 Oct 20. J Leukoc Biol. 2010. PMID: 19843574
-
Role of bone marrow stromal cells in the generation of human CD8+ regulatory T cells.Hum Immunol. 2008 Nov;69(11):755-9. doi: 10.1016/j.humimm.2008.08.278. Epub 2008 Sep 24. Hum Immunol. 2008. PMID: 18817823 Review.
-
Plasticity of T(reg) cells: is reprogramming of T(reg) cells possible in the presence of FOXP3?Int Immunopharmacol. 2011 May;11(5):555-60. doi: 10.1016/j.intimp.2010.11.024. Epub 2010 Nov 27. Int Immunopharmacol. 2011. PMID: 21115121 Review.
Cited by
-
Immunotype and immunohistologic characteristics of tumor-infiltrating immune cells are associated with clinical outcome in metastatic melanoma.Cancer Res. 2012 Mar 1;72(5):1070-80. doi: 10.1158/0008-5472.CAN-11-3218. Epub 2012 Jan 19. Cancer Res. 2012. PMID: 22266112 Free PMC article.
-
Emerging Skin T-Cell Functions in Response to Environmental Insults.J Invest Dermatol. 2017 Feb;137(2):288-294. doi: 10.1016/j.jid.2016.08.013. Epub 2016 Oct 23. J Invest Dermatol. 2017. PMID: 27784595 Free PMC article. Review.
-
Immune Checkpoints and Cellular Landscape of the Tumor Microenvironment in Non-Melanoma Skin Cancer (NMSC).Cells. 2024 Sep 26;13(19):1615. doi: 10.3390/cells13191615. Cells. 2024. PMID: 39404378 Free PMC article. Review.
-
Synergistic enhancement of CD8+ T cell-mediated tumor vaccine efficacy by an anti-transforming growth factor-beta monoclonal antibody.Clin Cancer Res. 2009 Nov 1;15(21):6560-9. doi: 10.1158/1078-0432.CCR-09-1066. Epub 2009 Oct 27. Clin Cancer Res. 2009. PMID: 19861451 Free PMC article.
-
Depletion of endogenous tumor-associated regulatory T cells improves the efficacy of adoptive cytotoxic T-cell immunotherapy in murine acute myeloid leukemia.Blood. 2009 Oct 29;114(18):3793-802. doi: 10.1182/blood-2009-03-208181. Epub 2009 Sep 1. Blood. 2009. PMID: 19724059 Free PMC article.
References
-
- Diepgen, T.L., and V. Mahler. 2002. The epidemiology of skin cancer. Br. J. Dermatol. 146:1–6. - PubMed
-
- Housman, T.S., S.R. Feldman, P.M. Williford, A.B. Fleischer Jr., N.D. Goldman, J.M. Acostamadiedo, and G.J. Chen. 2003. Skin cancer is among the most costly of all cancers to treat for the Medicare population. J. Am. Acad. Dermatol. 48:425–429. - PubMed
-
- Feldman, S.R., A.B. Fleischer Jr., and R.C. McConnell. 1998. Most common dermatologic problems identified by internists, 1990-1994. Arch. Intern. Med. 158:726–730. - PubMed
-
- Warino, L., M. Tusa, F. Camacho, H. Teuschler, A.B. Fleischer Jr., and S.R. Feldman. 2006. Frequency and cost of actinic keratosis treatment. Dermatol. Surg. 32:1045–1049. - PubMed
-
- Berg, D., and C.C. Otley. 2002. Skin cancer in organ transplant recipients: epidemiology, pathogenesis, and management. J. Am. Acad. Dermatol. 47:1–17. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

