Amyloid-beta oligomers set fire to inflammasomes and induce Alzheimer's pathology
- PMID: 18793350
- PMCID: PMC4514104
- DOI: 10.1111/j.1582-4934.2008.00496.x
Amyloid-beta oligomers set fire to inflammasomes and induce Alzheimer's pathology
Abstract
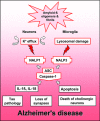
Genetic and molecular studies have confirmed the central role of amyloid-beta production and fibrillation in the pathogenesis of Alzheimer's disease (AD). However, the pathological pathways from amyloid-beta peptide oligomerization to the major pathological hallmarks of AD, such as neurofibrillary tangles, inflammation and loss of cholinergic neurons, are largely unknown. The innate immunity defence system utilizes pattern recognition receptors to respond to a variety of danger- and pathogen-associated molecular structures. Amyloid-beta oligomers and fibrils and their cellular effects can activate the innate immunity defence and induce inflammatory and apoptotic responses in human brain. Amyloid-beta oligomers can interfere with many aspects of neuronal membrane functions and can evoke potassium (K+) efflux from neurons. A low K+ concentration is a potent activator for the NALP1 inflammasomes, which then stimulate caspase-1 to cleave the proforms of IL-1beta and IL-18 cytokines. Interestingly, recent observations have demonstrated that amyloid-beta fibrils can activate NALP3 inflammasomes Via the lysosomal damage in mouse microglia. We will review here the activation mechanisms of NALP inflammasomes in neurons and microglia and several downstream effects in brain demonstrating that toxic amyloid-beta oligomers and fibrils can light afire in inflammasomes and induce Alzheimer's pathology.
Figures
Similar articles
-
Inflammation in Alzheimer's disease: amyloid-beta oligomers trigger innate immunity defence via pattern recognition receptors.Prog Neurobiol. 2009 Feb;87(3):181-94. doi: 10.1016/j.pneurobio.2009.01.001. Prog Neurobiol. 2009. PMID: 19388207 Review.
-
Inflammasomes as therapeutic targets for Alzheimer's disease.Brain Pathol. 2017 Mar;27(2):223-234. doi: 10.1111/bpa.12478. Brain Pathol. 2017. PMID: 28009077 Free PMC article. Review.
-
Autophagy in microglia degrades extracellular β-amyloid fibrils and regulates the NLRP3 inflammasome.Autophagy. 2014 Oct 1;10(10):1761-75. doi: 10.4161/auto.29647. Epub 2014 Jul 22. Autophagy. 2014. PMID: 25126727 Free PMC article.
-
Inflammasomes: guardians of cytosolic sanctity.Immunol Rev. 2009 Jan;227(1):95-105. doi: 10.1111/j.1600-065X.2008.00730.x. Immunol Rev. 2009. PMID: 19120479 Review.
-
Soluble Aβ oligomers and protofibrils induce NLRP3 inflammasome activation in microglia.J Neurochem. 2020 Dec;155(6):650-661. doi: 10.1111/jnc.14945. Epub 2020 Jan 30. J Neurochem. 2020. PMID: 31872431
Cited by
-
Expression of IL-1β in rhesus EAE and MS lesions is mainly induced in the CNS itself.J Neuroinflammation. 2016 Jun 6;13(1):138. doi: 10.1186/s12974-016-0605-8. J Neuroinflammation. 2016. PMID: 27266875 Free PMC article.
-
Cis-urocanic acid suppresses UV-B-induced interleukin-6 and -8 secretion and cytotoxicity in human corneal and conjunctival epithelial cells in vitro.Mol Vis. 2009 Sep 8;15:1799-805. Mol Vis. 2009. PMID: 19753313 Free PMC article.
-
Inhibition of hyperhomocysteinemia-induced inflammasome activation and glomerular sclerosis by NLRP3 gene deletion.Cell Physiol Biochem. 2014;34(3):829-41. doi: 10.1159/000363046. Epub 2014 Aug 20. Cell Physiol Biochem. 2014. PMID: 25171193 Free PMC article.
-
Deletion of p75NTR rescues the synaptic but not the inflammatory status in the brain of a mouse model for Alzheimer's disease.Front Mol Neurosci. 2023 May 5;16:1163087. doi: 10.3389/fnmol.2023.1163087. eCollection 2023. Front Mol Neurosci. 2023. PMID: 37213691 Free PMC article.
-
Targeting NADPH oxidase and phospholipases A2 in Alzheimer's disease.Mol Neurobiol. 2010 Jun;41(2-3):73-86. doi: 10.1007/s12035-010-8107-7. Epub 2010 Mar 2. Mol Neurobiol. 2010. PMID: 20195796 Free PMC article. Review.
References
-
- Tanzi RE, Bertram L. Twenty years of the Alzheimer's disease amyloid hypothesis: a genetic perspective. Cell. 2005;120:545–55. - PubMed
-
- Haas C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid β-peptide. Nature Rev Mol Cell Biol. 2007;8:101–12. - PubMed
-
- Walsh DM, Selkoe DJ. Aβ oligomers – a decade of discovery. J Neurochem. 2007;101:1172–84. - PubMed
-
- Williamson R, Usardl A, Hanger DP, Anderton BH. Membrane-bound β-amyloid oligomers are recruited into lipid rafts by a fyn-dependent mechanism. FASEB J. 2008;22:1552–9. - PubMed
-
- De Felice FG, Velasco PT, Lambert MP, Viola K, Fernandez SJ, Ferrelra ST, Klein WT. Aβ oligomers induce neuronal oxidative stress through an N-methyl-D-aspartate receptor-dependent mechanism that is blocked by the Alzheimer drug memantine. J Biol Chem. 2007;282:11590–601. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

