The histone H3K79 methyltransferase Dot1L is essential for mammalian development and heterochromatin structure
- PMID: 18787701
- PMCID: PMC2527135
- DOI: 10.1371/journal.pgen.1000190
The histone H3K79 methyltransferase Dot1L is essential for mammalian development and heterochromatin structure
Abstract
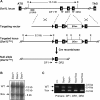
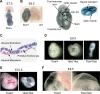
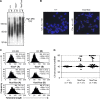
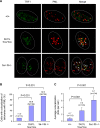
Dot1 is an evolutionarily conserved histone methyltransferase specific for lysine 79 of histone H3 (H3K79). In Saccharomyces cerevisiae, Dot1-mediated H3K79 methylation is associated with telomere silencing, meiotic checkpoint control, and DNA damage response. The biological function of H3K79 methylation in mammals, however, remains poorly understood. Using gene targeting, we generated mice deficient for Dot1L, the murine Dot1 homologue. Dot1L-deficient embryos show multiple developmental abnormalities, including growth impairment, angiogenesis defects in the yolk sac, and cardiac dilation, and die between 9.5 and 10.5 days post coitum. To gain insights into the cellular function of Dot1L, we derived embryonic stem (ES) cells from Dot1L mutant blastocysts. Dot1L-deficient ES cells show global loss of H3K79 methylation as well as reduced levels of heterochromatic marks (H3K9 di-methylation and H4K20 tri-methylation) at centromeres and telomeres. These changes are accompanied by aneuploidy, telomere elongation, and proliferation defects. Taken together, these results indicate that Dot1L and H3K79 methylation play important roles in heterochromatin formation and in embryonic development.
Conflict of interest statement
All authors except AB, SG, and YZ are employees of Novartis Institutes for Biomedical Research.
Figures

Similar articles
-
The histone methyltransferase Dot1/DOT1L as a critical regulator of the cell cycle.Cell Cycle. 2014;13(5):726-38. doi: 10.4161/cc.28104. Epub 2014 Feb 6. Cell Cycle. 2014. PMID: 24526115 Free PMC article. Review.
-
Dot1L mediated histone H3 lysine79 methylation is essential to meiosis progression in mouse oocytes.Neuro Endocrinol Lett. 2014;35(6):523-30. Neuro Endocrinol Lett. 2014. PMID: 25433842
-
DOT1L/KMT4 recruitment and H3K79 methylation are ubiquitously coupled with gene transcription in mammalian cells.Mol Cell Biol. 2008 Apr;28(8):2825-39. doi: 10.1128/MCB.02076-07. Epub 2008 Feb 19. Mol Cell Biol. 2008. PMID: 18285465 Free PMC article.
-
A charge-based interaction between histone H4 and Dot1 is required for H3K79 methylation and telomere silencing: identification of a new trans-histone pathway.Genes Dev. 2007 Aug 15;21(16):2018-29. doi: 10.1101/gad.1560607. Epub 2007 Aug 3. Genes Dev. 2007. PMID: 17675446 Free PMC article.
-
The diverse functions of Dot1 and H3K79 methylation.Genes Dev. 2011 Jul 1;25(13):1345-58. doi: 10.1101/gad.2057811. Genes Dev. 2011. PMID: 21724828 Free PMC article. Review.
Cited by
-
Implication of posttranslational histone modifications in nucleotide excision repair.Int J Mol Sci. 2012 Sep 28;13(10):12461-86. doi: 10.3390/ijms131012461. Int J Mol Sci. 2012. PMID: 23202908 Free PMC article. Review.
-
Sound of silence: the properties and functions of repressive Lys methyltransferases.Nat Rev Mol Cell Biol. 2015 Aug;16(8):499-513. doi: 10.1038/nrm4029. Nat Rev Mol Cell Biol. 2015. PMID: 26204160 Review.
-
Chromatin-modifying enzymes as modulators of reprogramming.Nature. 2012 Mar 4;483(7391):598-602. doi: 10.1038/nature10953. Nature. 2012. PMID: 22388813 Free PMC article.
-
Pluripotency of embryonic stem cells lacking clathrin-mediated endocytosis cannot be rescued by restoring cellular stiffness.J Biol Chem. 2020 Dec 4;295(49):16888-16896. doi: 10.1074/jbc.AC120.014343. Epub 2020 Oct 21. J Biol Chem. 2020. PMID: 33087446 Free PMC article.
-
siRNA-mediated methylation of Arabidopsis telomeres.PLoS Genet. 2010 Jun 10;6(6):e1000986. doi: 10.1371/journal.pgen.1000986. PLoS Genet. 2010. PMID: 20548962 Free PMC article.
References
-
- Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074–1080. - PubMed
-
- Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. - PubMed
-
- Feng Q, Wang H, Ng HH, Erdjument-Bromage H, Tempst P, et al. Methylation of H3-lysine 79 is mediated by a new family of HMTases without a SET domain. Curr Biol. 2002;12:1052–1058. - PubMed
-
- Janzen CJ, Hake SB, Lowell JE, Cross GA. Selective di- or trimethylation of histone H3 lysine 76 by two DOT1 homologs is important for cell cycle regulation in Trypanosoma brucei. Mol Cell. 2006;23:497–507. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

