Neutrophil primary granule proteins HBP and HNP1-3 boost bacterial phagocytosis by human and murine macrophages
- PMID: 18787642
- PMCID: PMC2532980
- DOI: 10.1172/JCI35740
Neutrophil primary granule proteins HBP and HNP1-3 boost bacterial phagocytosis by human and murine macrophages
Abstract
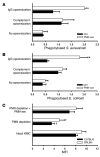
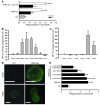
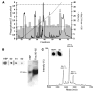
In acute inflammation, infiltrating polymorphonuclear leukocytes (also known as PMNs) release preformed granule proteins having multitudinous effects on the surrounding environment. Here we present what we believe to be a novel role for PMN-derived proteins in bacterial phagocytosis by both human and murine macrophages. Exposure of macrophages to PMN secretion markedly enhanced phagocytosis of IgG-opsonized Staphylococcus aureus both in vitro and in murine models in vivo. PMN secretion activated macrophages, resulting in upregulation of the Fcgamma receptors CD32 and CD64, which then mediated the enhanced phagocytosis of IgG-opsonized bacteria. The phagocytosis-stimulating activity within the PMN secretion was found to be due to proteins released from PMN primary granules; thorough investigation revealed heparin-binding protein (HBP) and human neutrophil peptides 1-3 (HNP1-3) as the mediators of the macrophage response to PMN secretion. The use of blocking antibodies and knockout mice revealed that HBP acts via beta2 integrins, but the receptor for HNP1-3 remained unclear. Mechanistically, HBP and HNP1-3 triggered macrophage release of TNF-alpha and IFN-gamma, which acted in an autocrine loop to enhance expression of CD32 and CD64 and thereby enhance phagocytosis. Thus, we attribute what may be a novel role for PMN granule proteins in regulating the immune response to bacterial infections.
Figures



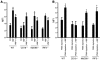
Similar articles
-
Role of neutrophil Fc gamma RIIa (CD32) and Fc gamma RIIIb (CD16) polymorphic forms in phagocytosis of human IgG1- and IgG3-opsonized bacteria and erythrocytes.Immunology. 1994 Dec;83(4):624-30. Immunology. 1994. PMID: 7875742 Free PMC article.
-
Rac2-deficient murine macrophages have selective defects in superoxide production and phagocytosis of opsonized particles.J Immunol. 2004 Nov 15;173(10):5971-9. doi: 10.4049/jimmunol.173.10.5971. J Immunol. 2004. PMID: 15528331
-
Neutrophil secretion products regulate anti-bacterial activity in monocytes and macrophages.Clin Exp Immunol. 2008 Jan;151(1):139-45. doi: 10.1111/j.1365-2249.2007.03532.x. Epub 2007 Dec 7. Clin Exp Immunol. 2008. PMID: 17991288 Free PMC article.
-
Neutrophil granule proteins tune monocytic cell function.Trends Immunol. 2009 Nov;30(11):538-46. doi: 10.1016/j.it.2009.06.006. Epub 2009 Aug 21. Trends Immunol. 2009. PMID: 19699683 Review.
-
Direct and alternative antimicrobial mechanisms of neutrophil-derived granule proteins.J Mol Med (Berl). 2009 Dec;87(12):1157-64. doi: 10.1007/s00109-009-0508-6. Epub 2009 Jul 31. J Mol Med (Berl). 2009. PMID: 19641860 Review.
Cited by
-
NETosis and NADPH oxidase: at the intersection of host defense, inflammation, and injury.Front Immunol. 2013 Mar 1;4:45. doi: 10.3389/fimmu.2013.00045. eCollection 2013. Front Immunol. 2013. PMID: 23459634 Free PMC article.
-
Poly(ethylene glycol)-containing hydrogels promote the release of primary granules from human blood-derived polymorphonuclear leukocytes.J Biomed Mater Res A. 2014 Dec;102(12):4252-61. doi: 10.1002/jbm.a.35101. Epub 2014 Feb 13. J Biomed Mater Res A. 2014. PMID: 24497370 Free PMC article.
-
Neutrophils as protagonists and targets in chronic inflammation.Nat Rev Immunol. 2017 Apr;17(4):248-261. doi: 10.1038/nri.2017.10. Epub 2017 Mar 13. Nat Rev Immunol. 2017. PMID: 28287106 Review.
-
Neutrophil-T cell crosstalk and the control of the host inflammatory response.Immunol Rev. 2023 Mar;314(1):36-49. doi: 10.1111/imr.13162. Epub 2022 Nov 3. Immunol Rev. 2023. PMID: 36326214 Free PMC article. Review.
-
The opioid peptide dynorphin A induces leukocyte responses via integrin Mac-1 (αMβ2, CD11b/CD18).Mol Pain. 2015 Jun 3;11:33. doi: 10.1186/s12990-015-0027-0. Mol Pain. 2015. PMID: 26036990 Free PMC article.
References
-
- Witko-Sarsat V., Rieu P., Descamps-Latscha B., Lesavre P., Halbwachs-Mecarelli L. Neutrophils: molecules, functions and pathophysiological aspects. Lab. Invest. . 2000;80:617–653. - PubMed
-
- Borregaard N., Cowland J.B. Granules of the human neutrophilic polymorphonuclear leukocyte. Blood. 1997;89:3503–3521. - PubMed
-
- Shiohara M., et al. Phenotypic and functional alterations of peripheral blood monocytes in neutrophil-specific granule deficiency. J. Leukoc. Biol. 2004;75:190–197. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

