Incorporation of membrane-anchored flagellin into influenza virus-like particles enhances the breadth of immune responses
- PMID: 18786995
- PMCID: PMC2583664
- DOI: 10.1128/JVI.01076-08
Incorporation of membrane-anchored flagellin into influenza virus-like particles enhances the breadth of immune responses
Abstract
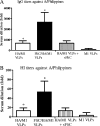
We have designed a membrane-anchored form of the Toll-like receptor 5 ligand flagellin, the major proinflammatory determinant of enteropathogenic Salmonella, which was found to be glycosylated and expressed on cell surfaces. A chimeric influenza virus-like particle (cVLP) vaccine candidate containing A/PR8/34 (H(1)N(1)) hemagglutinin (HA), matrix protein (M1), and the modified flagellin as a molecular adjuvant was produced. The immunogenicity, including the serum antibody levels and cellular immune responses, and the protective efficacy against homologous and heterologous live virus challenge of the resulting VLPs were tested after intramuscular administration in a mouse model. The results demonstrated that flagellin-containing VLPs elicited higher specific immunoglobulin G (IgG) responses than standard HA and M1 VLPs, indicating the adjuvant effect of flagellin. Enhanced IgG2a and IgG2b but not IgG1 responses were observed with flagellin-containing VLPs, illuminating the activation of Th1 class immunity. The adjuvant effects of flagellin were also reflected by enhanced specific cellular responses revealed by the secretion of cytokines by freshly isolated splenocyte cultures when stimulated with pools of major histocompatibility complex class I or II peptides. When immunized mice were challenged with homologous live PR8 virus, complete protection was observed for both the standard and cVLP groups. However, when a heterosubtypic A/Philippines (H(3)N(2)) virus was used for challenge, all of the standard VLP group lost at least 25% of body weight, reaching the experimental endpoint. In contrast, for the cVLP group, 67% of mice survived the challenge infection. These results reveal that cVLPs designed by incorporating flagellin as a membrane-anchored adjuvant induce enhanced cross-protective heterosubtypic immune responses. They also indicate that such cVLP vaccines are a promising new approach for protection against pandemic influenza viruses.
Figures

Similar articles
-
Intranasal immunization with influenza VLPs incorporating membrane-anchored flagellin induces strong heterosubtypic protection.PLoS One. 2010 Nov 29;5(11):e13972. doi: 10.1371/journal.pone.0013972. PLoS One. 2010. PMID: 21124769 Free PMC article.
-
Inclusion of membrane-anchored LTB or flagellin protein in H5N1 virus-like particles enhances protective responses following intramuscular and oral immunization of mice.Vaccine. 2018 Sep 25;36(40):5990-5998. doi: 10.1016/j.vaccine.2018.08.053. Epub 2018 Aug 30. Vaccine. 2018. PMID: 30172635
-
Synthetic Toll-like receptor 4 (TLR4) and TLR7 ligands as influenza virus vaccine adjuvants induce rapid, sustained, and broadly protective responses.J Virol. 2015 Mar;89(6):3221-35. doi: 10.1128/JVI.03337-14. Epub 2015 Jan 7. J Virol. 2015. PMID: 25568203 Free PMC article.
-
Virus-like particle vaccine induces protective immunity against homologous and heterologous strains of influenza virus.J Virol. 2007 Apr;81(7):3514-24. doi: 10.1128/JVI.02052-06. Epub 2007 Jan 24. J Virol. 2007. PMID: 17251294 Free PMC article.
-
Enhanced influenza virus-like particle vaccines containing the extracellular domain of matrix protein 2 and a Toll-like receptor ligand.Clin Vaccine Immunol. 2012 Aug;19(8):1119-25. doi: 10.1128/CVI.00153-12. Epub 2012 May 30. Clin Vaccine Immunol. 2012. PMID: 22647270 Free PMC article.
Cited by
-
Engineered Nanoparticle Applications for Recombinant Influenza Vaccines.Mol Pharm. 2021 Feb 1;18(2):576-592. doi: 10.1021/acs.molpharmaceut.0c00383. Epub 2020 Aug 17. Mol Pharm. 2021. PMID: 32787280 Free PMC article. Review.
-
The adjuvant activity of alphavirus replicons is enhanced by incorporating the microbial molecule flagellin into the replicon.PLoS One. 2013 Jun 13;8(6):e65964. doi: 10.1371/journal.pone.0065964. Print 2013. PLoS One. 2013. PMID: 23785460 Free PMC article.
-
Salmonella enterica serovar enteritidis core O polysaccharide conjugated to H:g,m flagellin as a candidate vaccine for protection against invasive infection with S. enteritidis.Infect Immun. 2011 Oct;79(10):4240-9. doi: 10.1128/IAI.05484-11. Epub 2011 Aug 1. Infect Immun. 2011. PMID: 21807909 Free PMC article.
-
A scalable method for biochemical purification of Salmonella flagellin.Protein Expr Purif. 2014 Oct;102:1-7. doi: 10.1016/j.pep.2014.07.005. Epub 2014 Jul 19. Protein Expr Purif. 2014. PMID: 25050462 Free PMC article.
-
A consensus-hemagglutinin-based vaccine delivered by an attenuated Salmonella mutant protects chickens against heterologous H7N1 influenza virus.Oncotarget. 2017 Jun 13;8(24):38780-38792. doi: 10.18632/oncotarget.16353. Oncotarget. 2017. PMID: 28418904 Free PMC article.
References
-
- Ben-Yedidia, T., and R. Arnon. 1998. Effect of pre-existing carrier immunity on the efficacy of synthetic influenza vaccine. Immunol. Lett. 649-15. - PubMed
-
- Bright, R. A., D. M. Carter, S. Daniluk, F. R. Toapanta, A. Ahmad, V. Gavrilov, M. Massare, P. Pushko, N. Mytle, T. Rowe, G. Smith, and T. M. Ross. 2007. Influenza virus-like particles elicit broader immune responses than whole virion inactivated influenza virus or recombinant hemagglutinin. Vaccine 253871-3878. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

