p75(NTR) mediates ephrin-A reverse signaling required for axon repulsion and mapping
- PMID: 18786358
- PMCID: PMC2677386
- DOI: 10.1016/j.neuron.2008.07.032
p75(NTR) mediates ephrin-A reverse signaling required for axon repulsion and mapping
Abstract
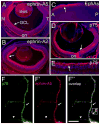
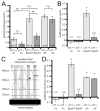
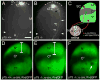
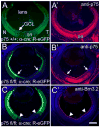
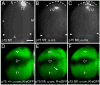
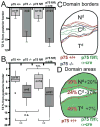
Reverse signaling by ephrin-As upon binding EphAs controls axon guidance and mapping. Ephrin-As are GPI-anchored to the membrane, requiring that they complex with transmembrane proteins that transduce their signals. We show that the p75 neurotrophin receptor (NTR) serves this role in retinal axons. p75(NTR) and ephrin-A colocalize within caveolae along retinal axons and form a complex required for Fyn phosphorylation upon binding EphAs, activating a signaling pathway leading to cytoskeletal changes. In vitro, retinal axon repulsion to EphAs by ephrin-A reverse signaling requires p75(NTR), but repulsion to ephrin-As by EphA forward signaling does not. Constitutive and retina-specific p75(NTR) knockout mice have aberrant anterior shifts in retinal axon terminations in superior colliculus, consistent with diminished repellent activity mediated by graded ephrin-A reverse signaling induced by graded collicular EphAs. We conclude that p75(NTR) is a signaling partner for ephrin-As and the ephrin-A- p75(NTR) complex reverse signals to mediate axon repulsion required for guidance and mapping.
Figures



Similar articles
-
EphrinA6 on chick retinal axons is a key component for p75(NTR)-dependent axon repulsion and TrkB-dependent axon branching.Mol Cell Neurosci. 2011 Jun;47(2):131-6. doi: 10.1016/j.mcn.2011.03.008. Epub 2011 Apr 2. Mol Cell Neurosci. 2011. PMID: 21463686 Free PMC article.
-
Regulation of axonal EphA4 forward signaling is involved in the effect of EphA3 on chicken retinal ganglion cell axon growth during retinotectal mapping.Exp Eye Res. 2019 Jan;178:46-60. doi: 10.1016/j.exer.2018.09.007. Epub 2018 Sep 18. Exp Eye Res. 2019. PMID: 30237102
-
New model of retinocollicular mapping predicts the mechanisms of axonal competition and explains the role of reverse molecular signaling during development.J Neurosci. 2012 Jul 11;32(28):9755-68. doi: 10.1523/JNEUROSCI.6180-11.2012. J Neurosci. 2012. PMID: 22787061 Free PMC article.
-
Eph and ephrin signaling in the formation of topographic maps.Semin Cell Dev Biol. 2012 Feb;23(1):7-15. doi: 10.1016/j.semcdb.2011.10.026. Epub 2011 Oct 24. Semin Cell Dev Biol. 2012. PMID: 22044886 Free PMC article. Review.
-
Visual map development: bidirectional signaling, bifunctional guidance molecules, and competition.Cold Spring Harb Perspect Biol. 2010 Nov;2(11):a001768. doi: 10.1101/cshperspect.a001768. Epub 2010 Sep 29. Cold Spring Harb Perspect Biol. 2010. PMID: 20880989 Free PMC article. Review.
Cited by
-
Dynamic plasticity: the role of glucocorticoids, brain-derived neurotrophic factor and other trophic factors.Neuroscience. 2013 Jun 3;239:214-27. doi: 10.1016/j.neuroscience.2012.08.034. Epub 2012 Aug 23. Neuroscience. 2013. PMID: 22922121 Free PMC article. Review.
-
Molecular complexity of visual mapping: a challenge for regenerating therapy.Neural Regen Res. 2020 Mar;15(3):382-389. doi: 10.4103/1673-5374.266044. Neural Regen Res. 2020. PMID: 31571645 Free PMC article. Review.
-
A Subtle Network Mediating Axon Guidance: Intrinsic Dynamic Structure of Growth Cone, Attractive and Repulsive Molecular Cues, and the Intermediate Role of Signaling Pathways.Neural Plast. 2019 Apr 14;2019:1719829. doi: 10.1155/2019/1719829. eCollection 2019. Neural Plast. 2019. PMID: 31097955 Free PMC article. Review.
-
Eph/ephrin molecules--a hub for signaling and endocytosis.Genes Dev. 2010 Nov 15;24(22):2480-92. doi: 10.1101/gad.1973910. Genes Dev. 2010. PMID: 21078817 Free PMC article. Review.
-
Guidance from above: common cues direct distinct signaling outcomes in vascular and neural patterning.Trends Cell Biol. 2009 Mar;19(3):99-110. doi: 10.1016/j.tcb.2009.01.001. Epub 2009 Feb 4. Trends Cell Biol. 2009. PMID: 19200729 Free PMC article. Review.
References
-
- Alsina B, Vu T, Cohen-Cory S. Visualizing synapse formation in arborizing optic axons in vivo: dynamics and modulation by BDNF. Nature Neurosci. 2001;4:1093–1101. - PubMed
-
- Barker PA. p75NTR is positively promiscuous: novel partners and new insights. Neuron. 2004;42:529–533. - PubMed
-
- Baumer N, Marquardt T, Stoykova A, Ashery-Padan R, Chowdhury K, Gruss P. Pax6 is required for establishing naso-temporal and dorsal characteristics of the optic vesicle. Development. 2002;129:4535–4545. - PubMed
-
- Brown A, Yates PA, Burrola P, Ortuño D, Vaidya A, Jessell TM, Pfaff SL, O’Leary DDM, Lemke G. Topographic mapping from the retina to the midbrain is controlled by relative but not absolute levels of EphA receptor signaling. Cell. 2000;7:77–88. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

