Resistance to RevM10 inhibition reflects a conformational switch in the HIV-1 Rev response element
- PMID: 18776047
- PMCID: PMC2567145
- DOI: 10.1073/pnas.0804461105
Resistance to RevM10 inhibition reflects a conformational switch in the HIV-1 Rev response element
Abstract
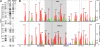
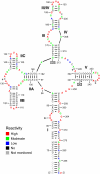
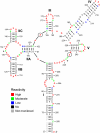
Nuclear export of certain HIV-1 mRNAs requires an interaction between the viral Rev protein and the Rev response element (RRE), a structured element located in the Env region of its RNA genome. This interaction is an attractive target for both drug design and gene therapy, exemplified by RevM10, a transdominant negative protein that, when introduced into host cells, disrupts viral mRNA export. However, two silent G->A mutations in the RRE (RRE61) confer RevM10 resistance, which prompted us to examine RRE structure using a novel chemical probing strategy. Variations in region III/IV/V of mutant RNAs suggest a stepwise rearrangement to RevM10 resistance. Mass spectrometry was used to directly assess Rev "loading" onto RRE and its variants, indicating that this is unaffected by RNA structural changes. Similarity in chemical footprints with mutant protein implicates additional host factors in RevM10 resistance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
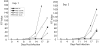

Similar articles
-
Functional analyses reveal extensive RRE plasticity in primary HIV-1 sequences selected under selective pressure.PLoS One. 2014 Aug 29;9(8):e106299. doi: 10.1371/journal.pone.0106299. eCollection 2014. PLoS One. 2014. PMID: 25170621 Free PMC article.
-
Evolution of the HIV-1 Rev Response Element during Natural Infection Reveals Nucleotide Changes That Correlate with Altered Structure and Increased Activity over Time.J Virol. 2019 May 15;93(11):e02102-18. doi: 10.1128/JVI.02102-18. Print 2019 Jun 1. J Virol. 2019. PMID: 30867301 Free PMC article.
-
Selection and characterization of human immunodeficiency virus type 1 mutants that are resistant to inhibition by the transdominant negative RevM10 protein.J Virol. 1999 Jul;73(7):5741-7. doi: 10.1128/JVI.73.7.5741-5747.1999. J Virol. 1999. PMID: 10364325 Free PMC article.
-
HIV Rev Assembly on the Rev Response Element (RRE): A Structural Perspective.Viruses. 2015 Jun 12;7(6):3053-75. doi: 10.3390/v7062760. Viruses. 2015. PMID: 26075509 Free PMC article. Review.
-
[HIV-1 Rev and related inhibitors].Yao Xue Xue Bao. 2007 Apr;42(4):347-51. Yao Xue Xue Bao. 2007. PMID: 17633198 Review. Chinese.
Cited by
-
An unusual topological structure of the HIV-1 Rev response element.Cell. 2013 Oct 24;155(3):594-605. doi: 10.1016/j.cell.2013.10.008. Epub 2013 Oct 24. Cell. 2013. PMID: 24243017 Free PMC article.
-
Single-nucleotide changes in the HIV Rev-response element mediate resistance to compounds that inhibit Rev function.J Virol. 2011 Apr;85(8):3940-9. doi: 10.1128/JVI.02683-10. Epub 2011 Feb 2. J Virol. 2011. PMID: 21289114 Free PMC article.
-
HIV-based lentiviral vectors: origin and sequence differences.Mol Ther Methods Clin Dev. 2021 Mar 27;21:451-465. doi: 10.1016/j.omtm.2021.03.018. eCollection 2021 Jun 11. Mol Ther Methods Clin Dev. 2021. PMID: 33981779 Free PMC article.
-
The HIV-1 Rev response element: an RNA scaffold that directs the cooperative assembly of a homo-oligomeric ribonucleoprotein complex.RNA Biol. 2012 Jan;9(1):6-11. doi: 10.4161/rna.9.1.18178. Epub 2012 Jan 1. RNA Biol. 2012. PMID: 22258145 Free PMC article.
-
Functional analyses reveal extensive RRE plasticity in primary HIV-1 sequences selected under selective pressure.PLoS One. 2014 Aug 29;9(8):e106299. doi: 10.1371/journal.pone.0106299. eCollection 2014. PLoS One. 2014. PMID: 25170621 Free PMC article.
References
-
- Fakuda M, et al. CRM1 is responsible for intracellular transport mediated by the nuclear export signal. Nature. 1997;390:308–311. - PubMed
-
- Fornerod M, Ohno M, Yoshida M, Mattaj M. CRM1 is an export receptor for leucine-rich nuclear export signals. Cell. 1997;90:1051–1060. - PubMed
-
- Arnold M, Nath A, Hauber J, Kehlenbach RH. Multiple importins function as nuclear transport receptors for the Rev protein of human immunodeficiency virus type 1. J Biol Chem. 2006;281:20883–20890. - PubMed
-
- Zapp ML, Green MR. Sequence-specific RNA binding by the HIV-1 Rev protein. Nature. 1989;342:714–716. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

