Ubiquitin binding and conjugation regulate the recruitment of Rabex-5 to early endosomes
- PMID: 18772883
- PMCID: PMC2567407
- DOI: 10.1038/emboj.2008.177
Ubiquitin binding and conjugation regulate the recruitment of Rabex-5 to early endosomes
Abstract
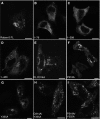
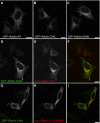
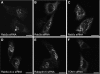
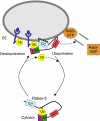
Rab GTPases and ubiquitination are critical regulators of transmembrane cargo sorting in endocytic and lysosomal targeting pathways. The endosomal protein Rabex-5 intersects these two layers of regulation by being both a guanine nucleotide exchange factor (GEF) for Rab5 and a substrate for ubiquitin (Ub) binding and conjugation. The ability of trafficking machinery components to bind ubiquitinated proteins is known to have a function in cargo sorting. Here, we demonstrate that Ub binding is essential for the recruitment of Rabex-5 from the cytosol to endosomes, independently of its GEF activity and of Rab5. We also show that monoubiquitinated Rabex-5 is enriched in the cytosol. These observations are consistent with a model whereby a cycle of Ub binding and monoubiquitination regulates the association of Rabex-5 with endosomes.
Figures

Similar articles
-
Spatiotemporal regulation of the ubiquitinated cargo-binding activity of Rabex-5 in the endocytic pathway.J Biol Chem. 2012 Nov 23;287(48):40586-97. doi: 10.1074/jbc.M112.411793. Epub 2012 Oct 9. J Biol Chem. 2012. PMID: 23048039 Free PMC article.
-
Rabaptin-5-independent membrane targeting and Rab5 activation by Rabex-5 in the cell.Mol Biol Cell. 2007 Oct;18(10):4119-28. doi: 10.1091/mbc.e07-02-0100. Epub 2007 Aug 15. Mol Biol Cell. 2007. PMID: 17699593 Free PMC article.
-
Rabex-5 is a Rab22 effector and mediates a Rab22-Rab5 signaling cascade in endocytosis.Mol Biol Cell. 2009 Nov;20(22):4720-9. doi: 10.1091/mbc.e09-06-0453. Epub 2009 Sep 16. Mol Biol Cell. 2009. PMID: 19759177 Free PMC article.
-
Who's in control? Principles of Rab GTPase activation in endolysosomal membrane trafficking and beyond.J Cell Biol. 2021 Sep 6;220(9):e202105120. doi: 10.1083/jcb.202105120. Epub 2021 Aug 12. J Cell Biol. 2021. PMID: 34383013 Free PMC article. Review.
-
[Effectors of GTPase Rab5 in endocytosis and signal transduction].Postepy Biochem. 2009;55(2):171-80. Postepy Biochem. 2009. PMID: 19824473 Review. Polish.
Cited by
-
Loss of HD-PTP function results in lipodystrophy, defective cellular signaling and altered lipid homeostasis.J Cell Sci. 2024 Sep 15;137(18):jcs262032. doi: 10.1242/jcs.262032. Epub 2024 Sep 27. J Cell Sci. 2024. PMID: 39155850
-
Stochastic activation and bistability in a Rab GTPase regulatory network.Proc Natl Acad Sci U S A. 2020 Mar 24;117(12):6540-6549. doi: 10.1073/pnas.1921027117. Epub 2020 Mar 11. Proc Natl Acad Sci U S A. 2020. PMID: 32161136 Free PMC article.
-
Regulation of early endosomes across eukaryotes: Evolution and functional homology of Vps9 proteins.Traffic. 2018 Jul;19(7):546-563. doi: 10.1111/tra.12570. Epub 2018 Apr 25. Traffic. 2018. PMID: 29603841 Free PMC article.
-
lncRNA HITT inhibits metastasis by attenuating Rab5-mediated endocytosis in lung adenocarcinoma.Mol Ther. 2022 Mar 2;30(3):1071-1088. doi: 10.1016/j.ymthe.2022.01.014. Epub 2022 Jan 8. Mol Ther. 2022. PMID: 35017116 Free PMC article.
-
Ubiquitin binding by the CUE domain promotes endosomal localization of the Rab5 GEF Vps9.Mol Biol Cell. 2015 Apr 1;26(7):1345-56. doi: 10.1091/mbc.E14-06-1156. Epub 2015 Feb 11. Mol Biol Cell. 2015. PMID: 25673804 Free PMC article.
References
-
- Amit I, Yakir L, Katz M, Zwang Y, Marmor MD, Citri A, Shtiegman K, Alroy I, Tuvia S, Reiss Y, Roubini E, Cohen M, Wides R, Bacharach E, Schubert U, Yarden Y (2004) Tal, a Tsg101-specific E3 ubiquitin ligase, regulates receptor endocytosis and retrovirus budding. Genes Dev 18: 1737–1752 - PMC - PubMed
-
- Brett TJ, Traub LM, Fremont DH (2002) Accessory protein recruitment motifs in clathrin-mediated endocytosis. Structure 10: 797–809 - PubMed
-
- Burkhard P, Stetefeld J, Strelkov SV (2001) Coiled coils: a highly versatile protein folding motif. Trends Cell Biol 11: 82–88 - PubMed
-
- Chyung JH, Selkoe DJ (2003) Inhibition of receptor-mediated endocytosis demonstrates generation of amyloid beta-protein at the cell surface. J Biol Chem 278: 51035–51043 - PubMed
-
- Clague MJ, Urbé S (2006) Endocytosis: the DUB version. Trends Cell Biol 16: 551–559 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

