Patients with relapsing-remitting multiple sclerosis have normal Treg function when cells expressing IL-7 receptor alpha-chain are excluded from the analysis
- PMID: 18769633
- PMCID: PMC2525698
- DOI: 10.1172/JCI35365
Patients with relapsing-remitting multiple sclerosis have normal Treg function when cells expressing IL-7 receptor alpha-chain are excluded from the analysis
Abstract
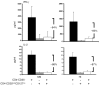
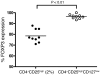
Multiple sclerosis (MS) is a chronic inflammatory disease that results in demyelination in the central nervous system, and a defect in the regulatory function of CD4+CD25high T cells has been implicated in the pathogenesis of the disease. Here, we reanalyzed the function of this T cell subset in patients with MS, but we depleted cells expressing IL-7 receptor alpha-chain (CD127), a marker recently described as present on activated T cells but not Tregs. Similar to other studies, we observed a marked defect in the suppressive function of unseparated CD4+CD25high T cells isolated from MS patients. However, when CD127(high) cells were removed from the CD4+CD25high population, patient and control cells inhibited T cell proliferation and cytokine production equally. Likewise, when the CD25 gate used to sort the cells was stringent enough to eliminate CD127high cells, CD4+CD25high T cells from patients with MS and healthy individuals had similar regulatory function. Additional analysis indicated that the CD127high cells within the CD4+CD25high T cell population from patients with MS appeared more proliferative and secreted more IFN-gamma and IL-2 than the same cells from healthy individuals. Taken together, we conclude that CD4+CD25highCD127low Tregs from MS patients and healthy individuals exhibit similar suppressive functions. The decreased inhibitory function of unfractioned CD4+CD25high cells previously observed might be due to abnormal activation of CD127high T cells in patients with MS.
Figures


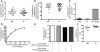

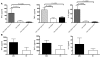
Similar articles
-
Secondary progressive in contrast to relapsing-remitting multiple sclerosis patients show a normal CD4+CD25+ regulatory T-cell function and FOXP3 expression.J Neurosci Res. 2006 Jun;83(8):1432-46. doi: 10.1002/jnr.20852. J Neurosci Res. 2006. PMID: 16583400
-
Reduced CD4+,CD25- T cell sensitivity to the suppressive function of CD4+,CD25high,CD127 -/low regulatory T cells in patients with active systemic lupus erythematosus.Arthritis Rheum. 2008 Jul;58(7):2120-30. doi: 10.1002/art.23556. Arthritis Rheum. 2008. PMID: 18576316
-
Reduced suppressive effect of CD4+CD25high regulatory T cells on the T cell immune response against myelin oligodendrocyte glycoprotein in patients with multiple sclerosis.Eur J Immunol. 2005 Nov;35(11):3343-52. doi: 10.1002/eji.200526065. Eur J Immunol. 2005. PMID: 16206232
-
Regulatory T cells fail to suppress CD4T+-bet+ T cells in relapsing multiple sclerosis patients.Immunology. 2009 Jul;127(3):418-28. doi: 10.1111/j.1365-2567.2008.02963.x. Epub 2008 Nov 7. Immunology. 2009. PMID: 19016907 Free PMC article.
-
Compromised CD4+ CD25(high) regulatory T-cell function in patients with relapsing-remitting multiple sclerosis is correlated with a reduced frequency of FOXP3-positive cells and reduced FOXP3 expression at the single-cell level.Immunology. 2008 Jan;123(1):79-89. doi: 10.1111/j.1365-2567.2007.02690.x. Epub 2007 Sep 25. Immunology. 2008. PMID: 17897326 Free PMC article.
Cited by
-
Regulatory Cell Populations in Relapsing-Remitting Multiple Sclerosis (RRMS) Patients: Effect of Disease Activity and Treatment Regimens.Int J Mol Sci. 2016 Aug 25;17(9):1398. doi: 10.3390/ijms17091398. Int J Mol Sci. 2016. PMID: 27571060 Free PMC article.
-
The PD-1 pathway in tolerance and autoimmunity.Immunol Rev. 2010 Jul;236:219-42. doi: 10.1111/j.1600-065X.2010.00923.x. Immunol Rev. 2010. PMID: 20636820 Free PMC article. Review.
-
Generation of RORγt+ Antigen-Specific T Regulatory 17 Cells from Foxp3+ Precursors in Autoimmunity.Cell Rep. 2017 Oct 3;21(1):195-207. doi: 10.1016/j.celrep.2017.09.021. Cell Rep. 2017. PMID: 28978473 Free PMC article.
-
A Perspective on Oral Immunotherapeutic Tools and Strategies for Autoimmune Disorders.Vaccines (Basel). 2023 May 27;11(6):1031. doi: 10.3390/vaccines11061031. Vaccines (Basel). 2023. PMID: 37376420 Free PMC article. Review.
-
The gut microbiome molecular mimicry piece in the multiple sclerosis puzzle.Front Immunol. 2022 Aug 15;13:972160. doi: 10.3389/fimmu.2022.972160. eCollection 2022. Front Immunol. 2022. PMID: 36045671 Free PMC article. Review.
References
-
- Sakaguchi S., Sakaguchi N., Asano M., Itoh M., Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 1995;155:1151–1164. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

