The heart of autophagy: deconstructing cardiac proteotoxicity
- PMID: 18769158
- PMCID: PMC2789566
- DOI: 10.4161/auto.6756
The heart of autophagy: deconstructing cardiac proteotoxicity
Abstract
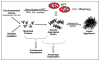
The heart is capable of robust structural remodeling, sometimes improving performance and sometimes leading to failure. Recent studies have uncovered a critical role for autophagy in disease-related remodeling of the cardiomyocyte. We have shown previously that hemodynamic load elicits a maladaptive autophagic response in cardiomyocytes which contributes to disease progression. In a recent study, we went on to demonstrate that protein aggregation is a proximal event triggering autophagic clearance mechanisms. The ubiquitin-proteasome-dependent pathways of protein clearance are similarly activated in parallel with processing of stress-induced protein aggregates into aggresomes and clearance through autophagy. These findings in the setting of pressure overload contrast with protein aggregation occurring in a model of protein chaperone malfunction in myocytes, where activation of autophagy is beneficial, antagonizing disease progression. Our findings situate heart disease stemming from environmental stress in the category of proteinopathy and raise important new questions regarding molecular events that elicit adaptive and maladaptive autophagy.
Figures
Similar articles
-
Autophagy in load-induced heart disease.Circ Res. 2008 Dec 5;103(12):1363-9. doi: 10.1161/CIRCRESAHA.108.186551. Circ Res. 2008. PMID: 19059838 Free PMC article. Review.
-
Intracellular protein aggregation is a proximal trigger of cardiomyocyte autophagy.Circulation. 2008 Jun 17;117(24):3070-8. doi: 10.1161/CIRCULATIONAHA.107.763870. Epub 2008 Jun 9. Circulation. 2008. PMID: 18541737 Free PMC article.
-
Impaired Protein Quality Control During Left Ventricular Remodeling in Mice With Cardiac Restricted Overexpression of Tumor Necrosis Factor.Circ Heart Fail. 2017 Dec;10(12):e004252. doi: 10.1161/CIRCHEARTFAILURE.117.004252. Circ Heart Fail. 2017. PMID: 29203562 Free PMC article.
-
Proteotoxicity: an underappreciated pathology in cardiac disease.J Mol Cell Cardiol. 2014 Jun;71:3-10. doi: 10.1016/j.yjmcc.2013.12.015. Epub 2013 Dec 28. J Mol Cell Cardiol. 2014. PMID: 24380730 Free PMC article. Review.
-
The ubiquitin-proteasome system in cardiac proteinopathy: a quality control perspective.Cardiovasc Res. 2010 Jan 15;85(2):253-62. doi: 10.1093/cvr/cvp287. Epub 2009 Aug 20. Cardiovasc Res. 2010. PMID: 19696071 Free PMC article. Review.
Cited by
-
Proteasome functional insufficiency in cardiac pathogenesis.Am J Physiol Heart Circ Physiol. 2011 Dec;301(6):H2207-19. doi: 10.1152/ajpheart.00714.2011. Epub 2011 Sep 23. Am J Physiol Heart Circ Physiol. 2011. PMID: 21949118 Free PMC article. Review.
-
Hold me tight: Role of the heat shock protein family of chaperones in cardiac disease.Circulation. 2010 Oct 26;122(17):1740-51. doi: 10.1161/CIRCULATIONAHA.110.942250. Circulation. 2010. PMID: 20975010 Free PMC article. Review. No abstract available.
-
HACE1-dependent protein degradation provides cardiac protection in response to haemodynamic stress.Nat Commun. 2014 Mar 11;5:3430. doi: 10.1038/ncomms4430. Nat Commun. 2014. PMID: 24614889 Free PMC article.
-
Autophagy in load-induced heart disease.Methods Enzymol. 2009;453:343-63. doi: 10.1016/S0076-6879(08)04017-2. Methods Enzymol. 2009. PMID: 19216915 Free PMC article.
-
Autophagy in load-induced heart disease.Circ Res. 2008 Dec 5;103(12):1363-9. doi: 10.1161/CIRCRESAHA.108.186551. Circ Res. 2008. PMID: 19059838 Free PMC article. Review.
References
-
- Hill JA, Olson EN. Cardiac plasticity. New Engl J Med. 2008;358:1370–80. - PubMed
-
- Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol. 2006;7:589–600. - PubMed
-
- Sybers HD, Ingwall J, DeLuca M. Autophagy in cardiac myocytes. Recent Adv Stud Cardiac Struct Metab. 1976;12:453–63. - PubMed
-
- Decker RS, Decker ML, Herring GH, Morton PC, Wildenthal K. Lysosomal vacuolar apparatus of cardiac myocytes in heart of starved and refed rabbits. J Mol Cell Cardiol. 1980;12:1175–89. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
