HCMV gH/gL/UL128-131 interferes with virus entry into epithelial cells: evidence for cell type-specific receptors
- PMID: 18768787
- PMCID: PMC2544588
- DOI: 10.1073/pnas.0804365105
HCMV gH/gL/UL128-131 interferes with virus entry into epithelial cells: evidence for cell type-specific receptors
Abstract
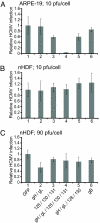
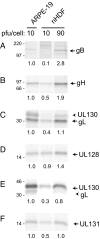
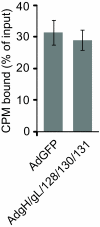
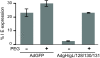
Human cytomegalovirus (HCMV) forms two different membrane protein complexes, gH/gL/gO and gH/gL/UL128/UL130/UL131, that function in different cell types. gH/gL/gO appears to be important for HCMV entry into or spread between fibroblasts, processes that occur at neutral pH. We demonstrated that HCMV entry into epithelial and endothelial cells requires gH/gL/UL128-131 and involves endocytosis and low pH. A complex of all five HCMV proteins, gH, gL, UL128, UL130, and UL131, is the functionally important mediator of this entry pathway into epithelial/endothelial cells. Here, we report that expression of gH/gL/UL128-131 in ARPE-19 epithelial cells causes the cells to be resistant to HCMV infection. Another HCMV glycoprotein, gB, did not interfere, and expression of all five gH/gL/UL128-131 proteins was required for this interference. gH/gL/UL128-131 interference was at the stage of virus entry into cells rather than the initial adsorption onto cell surfaces or after-entry defects. By contrast, expression of gH/gL/UL128-131 in primary human fibroblasts did not block HCMV infection. Previously, interference by retrovirus and herpes-simplex-virus entry mediators resulted from sequestration or obstruction of receptors. We concluded that epithelial cells express gH/gL/UL128-131 receptors that mediate HCMV entry. Fibroblasts either lack the gH/gL/UL128-131 receptors, the receptors are more numerous, or fibroblasts express other functional receptors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Human cytomegalovirus glycoprotein gO complexes with gH/gL, promoting interference with viral entry into human fibroblasts but not entry into epithelial cells.J Virol. 2011 Nov;85(22):11638-45. doi: 10.1128/JVI.05659-11. Epub 2011 Aug 31. J Virol. 2011. PMID: 21880752 Free PMC article.
-
Human cytomegalovirus TR strain glycoprotein O acts as a chaperone promoting gH/gL incorporation into virions but is not present in virions.J Virol. 2010 Mar;84(5):2597-609. doi: 10.1128/JVI.02256-09. Epub 2009 Dec 23. J Virol. 2010. PMID: 20032193 Free PMC article.
-
Characterization of the human cytomegalovirus gH/gL/UL128-131 complex that mediates entry into epithelial and endothelial cells.J Virol. 2008 Jan;82(1):60-70. doi: 10.1128/JVI.01910-07. Epub 2007 Oct 17. J Virol. 2008. PMID: 17942555 Free PMC article.
-
Human cytomegalovirus tropism for endothelial/epithelial cells: scientific background and clinical implications.Rev Med Virol. 2010 May;20(3):136-55. doi: 10.1002/rmv.645. Rev Med Virol. 2010. PMID: 20084641 Review.
-
Human Cytomegalovirus Cell Tropism and Host Cell Receptors.Vaccines (Basel). 2019 Jul 22;7(3):70. doi: 10.3390/vaccines7030070. Vaccines (Basel). 2019. PMID: 31336680 Free PMC article. Review.
Cited by
-
Monoclonal Antibodies to Different Components of the Human Cytomegalovirus (HCMV) Pentamer gH/gL/pUL128L and Trimer gH/gL/gO as well as Antibodies Elicited during Primary HCMV Infection Prevent Epithelial Cell Syncytium Formation.J Virol. 2016 Jun 24;90(14):6216-6223. doi: 10.1128/JVI.00121-16. Print 2016 Jul 15. J Virol. 2016. PMID: 27122579 Free PMC article.
-
HCMV gB shares structural and functional properties with gB proteins from other herpesviruses.Virology. 2013 Jan 20;435(2):239-49. doi: 10.1016/j.virol.2012.09.024. Epub 2012 Oct 22. Virology. 2013. PMID: 23089254 Free PMC article.
-
Stuck in the middle: structural insights into the role of the gH/gL heterodimer in herpesvirus entry.Curr Opin Virol. 2013 Feb;3(1):13-9. doi: 10.1016/j.coviro.2012.10.005. Epub 2012 Oct 26. Curr Opin Virol. 2013. PMID: 23107819 Free PMC article. Review.
-
Human Cytomegalovirus Envelope Protein gpUL132 Regulates Infectious Virus Production through Formation of the Viral Assembly Compartment.mBio. 2020 Sep 29;11(5):e02044-20. doi: 10.1128/mBio.02044-20. mBio. 2020. PMID: 32994323 Free PMC article.
-
Herpes virus fusion and entry: a story with many characters.Viruses. 2012 May;4(5):800-32. doi: 10.3390/v4050800. Epub 2012 May 10. Viruses. 2012. PMID: 22754650 Free PMC article. Review.
References
-
- Britt WJ. In: Cytomegaloviruses: Biology and Immunology. Reddehase M, editor. Norfolk, UK: Caister Academic; 2006. pp. 1–28.
-
- Pass RF. In: Fields Virology. Knipe DM, Howley PM, editors. Vol 2. Philadelphia: Lippincott Willliams & Davis; 2001. pp. 2675–2706.
-
- Landolfo S, Gariglio M, Gribaudo G, Lembo D. The human cytomegalovirus. Pharmacol Ther. 2003;98:269–297. - PubMed
-
- Sinzger C, et al. Fibroblasts, epithelial cells, endothelial cells, and smooth muscle cells are major targets of human cytomegalovirus infection in lung and gastrointestinal tissues. J Gen Virol. 1995;76(Pt 4):741–750. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

