Novel HPMA copolymer-bound constructs for combined tumor and mitochondrial targeting
- PMID: 18767867
- PMCID: PMC3129976
- DOI: 10.1021/mp800019g
Novel HPMA copolymer-bound constructs for combined tumor and mitochondrial targeting
Abstract
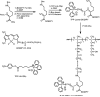
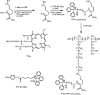
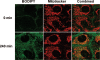
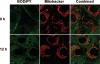
A wide variety of therapeutic agents may benefit by specifically directing them to the mitochondria in tumor cells. The current work aimed to design delivery systems that would enable a combination of tumor and mitochondrial targeting for such therapeutic entities. To this end, novel HPMA copolymer-based delivery systems that employ triphenylphosphonium (TPP) ions as mitochondriotropic agents were developed. Constructs were initially synthesized with fluorescent labels substituting for drug and were used for validation experiments. Microinjection and incubation experiments performed using these fluorescently labeled constructs confirmed the mitochondrial targeting ability. Subsequently, HPMA copolymer-drug conjugates were synthesized using a photosensitizer mesochlorin e 6 (Mce 6). Mitochondrial targeting of HPMA copolymer-bound Mce 6 enhanced cytotoxicity as compared to nontargeted HPMA copolymer-Mce 6 conjugates. Minor modifications may be required to adapt the current design and allow for tumor site-specific mitochondrial targeting of other therapeutic agents.
Figures
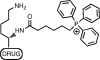
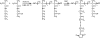
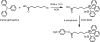

Similar articles
-
Combination chemotherapy and photodynamic therapy with fab' fragment targeted HPMA copolymer conjugates in human ovarian carcinoma cells.Mol Pharm. 2008 Sep-Oct;5(5):696-709. doi: 10.1021/mp800006e. Epub 2008 Aug 27. Mol Pharm. 2008. PMID: 18729468 Free PMC article.
-
Antitumor activity of N-(2-hydroxypropyl) methacrylamide copolymer-Mesochlorine e6 and adriamycin conjugates in combination treatments.Clin Cancer Res. 2000 Mar;6(3):1008-15. Clin Cancer Res. 2000. PMID: 10741728
-
Cooperativity between free and N-(2-hydroxypropyl) methacrylamide copolymer bound adriamycin and meso-chlorin e6 monoethylene diamine induced photodynamic therapy in human epithelial ovarian carcinoma in vitro.Int J Oncol. 1999 Jul;15(1):5-16. doi: 10.3892/ijo.15.1.5. Int J Oncol. 1999. PMID: 10375588
-
HPMA copolymer-cyclic RGD conjugates for tumor targeting.Adv Drug Deliv Rev. 2010 Feb 17;62(2):167-83. doi: 10.1016/j.addr.2009.11.027. Epub 2009 Dec 4. Adv Drug Deliv Rev. 2010. PMID: 19951733 Review.
-
Development of HPMA copolymer-anticancer conjugates: clinical experience and lessons learnt.Adv Drug Deliv Rev. 2009 Nov 12;61(13):1131-48. doi: 10.1016/j.addr.2009.05.007. Epub 2009 Aug 20. Adv Drug Deliv Rev. 2009. PMID: 19699249 Review.
Cited by
-
Polymer nanomedicines.Adv Drug Deliv Rev. 2020;156:40-64. doi: 10.1016/j.addr.2020.07.020. Epub 2020 Jul 28. Adv Drug Deliv Rev. 2020. PMID: 32735811 Free PMC article. Review.
-
Surface conjugation of triphenylphosphonium to target poly(amidoamine) dendrimers to mitochondria.Biomaterials. 2012 Jun;33(18):4773-82. doi: 10.1016/j.biomaterials.2012.03.032. Epub 2012 Apr 1. Biomaterials. 2012. PMID: 22469294 Free PMC article.
-
Polymer-drug conjugates: origins, progress to date and future directions.Adv Drug Deliv Rev. 2013 Jan;65(1):49-59. doi: 10.1016/j.addr.2012.10.014. Epub 2012 Nov 2. Adv Drug Deliv Rev. 2013. PMID: 23123294 Free PMC article. Review.
-
Biomaterials and drug delivery: past, present, and future.Mol Pharm. 2010 Aug 2;7(4):922-5. doi: 10.1021/mp1001813. Mol Pharm. 2010. PMID: 20672864 Free PMC article. No abstract available.
-
Intracellular trafficking and subcellular distribution of a large array of HPMA copolymers.Biomacromolecules. 2009 Jul 13;10(7):1704-14. doi: 10.1021/bm801514x. Epub 2009 May 21. Biomacromolecules. 2009. PMID: 21197960 Free PMC article.
References
-
- Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent SMANCS. Cancer Res. 1986;46:6387–6392. - PubMed
-
- Allen TM. Ligand-targeted therapeutics in anticancer therapy. Nat. Rev. Cancer. 2002;2:750–763. - PubMed
-
- Duncan R. The dawning era of polymer therapeutics. Nat. Rev. Drug Discovery. 2003;2:347–360. - PubMed
-
- Kopeček J, Kopečková P, Minko T, Lu Z. HPMA copolymeranticancer drug conjugates: design, activity, and mechanism of action. Eur. J. Pharm. Biopharm. 2000;50:61–81. - PubMed
-
- Shen WC, Ryser HJ. cis-Aconityl spacer between daunomycin and macromolecular carriers: a model of pH-sensitive linkage releasing drug from a lysosomotropic conjugate. Biochem. Biophys. Res. Commun. 1981;102:1048–1054. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

