Pathogen induction of CXCR4/TLR2 cross-talk impairs host defense function
- PMID: 18765807
- PMCID: PMC2533224
- DOI: 10.1073/pnas.0803852105
Pathogen induction of CXCR4/TLR2 cross-talk impairs host defense function
Abstract
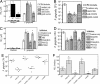
We report a mechanism of microbial evasion of Toll-like receptor (TLR)-mediated immunity that depends on CXCR4 exploitation. Specifically, the oral/systemic pathogen Porphyromonas gingivalis induces cross-talk between CXCR4 and TLR2 in human monocytes or mouse macrophages and undermines host defense. This is accomplished through its surface fimbriae, which induce CXCR4/TLR2 co-association in lipid rafts and interact with both receptors: Binding to CXCR4 induces cAMP-dependent protein kinase A (PKA) signaling, which in turn inhibits TLR2-mediated proinflammatory and antimicrobial responses to the pathogen. This outcome enables P. gingivalis to resist clearance in vitro and in vivo and thus to promote its adaptive fitness. However, a specific CXCR4 antagonist abrogates this immune evasion mechanism and offers a promising counterstrategy for the control of P. gingivalis periodontal or systemic infections.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
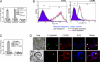
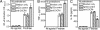
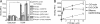
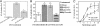
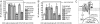
Similar articles
-
Mechanism and implications of CXCR4-mediated integrin activation by Porphyromonas gingivalis.Mol Oral Microbiol. 2013 Aug;28(4):239-49. doi: 10.1111/omi.12021. Epub 2013 Jan 21. Mol Oral Microbiol. 2013. PMID: 23331495 Free PMC article.
-
Fimbrial proteins of porphyromonas gingivalis mediate in vivo virulence and exploit TLR2 and complement receptor 3 to persist in macrophages.J Immunol. 2007 Aug 15;179(4):2349-58. doi: 10.4049/jimmunol.179.4.2349. J Immunol. 2007. PMID: 17675496
-
Macrophage-specific TLR2 signaling mediates pathogen-induced TNF-dependent inflammatory oral bone loss.J Immunol. 2013 Feb 1;190(3):1148-57. doi: 10.4049/jimmunol.1202511. Epub 2012 Dec 21. J Immunol. 2013. PMID: 23264656 Free PMC article.
-
Porphyromonas gingivalis mediated periodontal disease and atherosclerosis: disparate diseases with commonalities in pathogenesis through TLRs.Curr Pharm Des. 2007;13(36):3665-75. doi: 10.2174/138161207783018554. Curr Pharm Des. 2007. PMID: 18220804 Review.
-
Subversion of innate immunity by periodontopathic bacteria via exploitation of complement receptor-3.Adv Exp Med Biol. 2008;632:203-19. doi: 10.1007/978-0-387-78952-1_15. Adv Exp Med Biol. 2008. PMID: 19025124 Free PMC article. Review.
Cited by
-
Complement and dysbiosis in periodontal disease.Immunobiology. 2012 Nov;217(11):1111-6. doi: 10.1016/j.imbio.2012.07.007. Immunobiology. 2012. PMID: 22964237 Free PMC article. Review.
-
Porphyromonas gingivalis Fimbria-Induced Expression of Inflammatory Cytokines and Cyclooxygenase-2 in Mouse Macrophages and Its Inhibition by the Bioactive Compounds Fibronectin and Melatonin.ISRN Dent. 2012;2012:350859. doi: 10.5402/2012/350859. Epub 2012 Apr 1. ISRN Dent. 2012. PMID: 22545218 Free PMC article.
-
"Gum bug, leave my heart alone!"--epidemiologic and mechanistic evidence linking periodontal infections and atherosclerosis.J Dent Res. 2010 Sep;89(9):879-902. doi: 10.1177/0022034510375281. Epub 2010 Jul 16. J Dent Res. 2010. PMID: 20639510 Free PMC article. Review.
-
Exploring the neuroimmunopharmacology of opioids: an integrative review of mechanisms of central immune signaling and their implications for opioid analgesia.Pharmacol Rev. 2011 Sep;63(3):772-810. doi: 10.1124/pr.110.004135. Epub 2011 Jul 13. Pharmacol Rev. 2011. PMID: 21752874 Free PMC article. Review.
-
Location, location, location: is membrane partitioning everything when it comes to innate immune activation?Mediators Inflamm. 2011;2011:186093. doi: 10.1155/2011/186093. Epub 2011 Jun 21. Mediators Inflamm. 2011. PMID: 21765613 Free PMC article. Review.
References
-
- Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1:135–145. - PubMed
-
- Beutler B, et al. Genetic analysis of host resistance: Toll-like receptor signaling and immunity at large. Annu Rev Immunol. 2006;24:353–389. - PubMed
-
- Hajishengallis G, et al. Differential interactions of fimbriae and lipopolysaccharide from Porphyromonas gingivalis with the Toll-like receptor 2-centred pattern recognition apparatus. Cell Microbiol. 2006;8:1557–1570. - PubMed
-
- Triantafilou M, Brandenburg K, Gutsmann T, Seydel U, Triantafilou K. Innate recognition of bacteria: Engagement of multiple receptors. Crit Rev Immunol. 2002;22:251–268. - PubMed
-
- Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005;366:1809–1820. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

