Making an effort to listen: mechanical amplification in the ear
- PMID: 18760690
- PMCID: PMC2724262
- DOI: 10.1016/j.neuron.2008.07.012
Making an effort to listen: mechanical amplification in the ear
Abstract
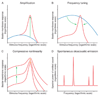
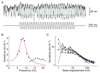
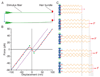
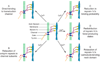
The inner ear's performance is greatly enhanced by an active process defined by four features: amplification, frequency selectivity, compressive nonlinearity, and spontaneous otoacoustic emission. These characteristics emerge naturally if the mechanoelectrical transduction process operates near a dynamical instability, the Hopf bifurcation, whose mathematical properties account for specific aspects of our hearing. The active process of nonmammalian tetrapods depends upon active hair-bundle motility, which emerges from the interaction of negative hair-bundle stiffness and myosin-based adaptation motors. Taken together, these phenomena explain the four characteristics of the ear's active process. In the high-frequency region of the mammalian cochlea, the active process is dominated instead by the phenomenon of electromotility, in which the cell bodies of outer hair cells extend and contract as the protein prestin alters its membrane surface area in response to changes in membrane potential.
Figures


Similar articles
-
The physics of hearing: fluid mechanics and the active process of the inner ear.Rep Prog Phys. 2014 Jul;77(7):076601. doi: 10.1088/0034-4885/77/7/076601. Epub 2014 Jul 9. Rep Prog Phys. 2014. PMID: 25006839 Review.
-
A critique of the critical cochlea: Hopf--a bifurcation--is better than none.J Neurophysiol. 2010 Sep;104(3):1219-29. doi: 10.1152/jn.00437.2010. Epub 2010 Jun 10. J Neurophysiol. 2010. PMID: 20538769 Free PMC article. Review.
-
How the ear's works work: mechanoelectrical transduction and amplification by hair cells.C R Biol. 2005 Feb;328(2):155-62. doi: 10.1016/j.crvi.2004.12.003. C R Biol. 2005. PMID: 15771001 Review.
-
Integrating the active process of hair cells with cochlear function.Nat Rev Neurosci. 2014 Sep;15(9):600-14. doi: 10.1038/nrn3786. Epub 2014 Aug 6. Nat Rev Neurosci. 2014. PMID: 25096182 Review.
-
Somatic motility and hair bundle mechanics, are both necessary for cochlear amplification?Hear Res. 2011 Mar;273(1-2):109-22. doi: 10.1016/j.heares.2010.03.094. Epub 2010 Apr 27. Hear Res. 2011. PMID: 20430075 Free PMC article.
Cited by
-
The effects and outcomes of electrolyte disturbances and asphyxia on newborns hearing.Int J Pediatr Otorhinolaryngol. 2013 Jul;77(7):1072-6. doi: 10.1016/j.ijporl.2013.03.031. Epub 2013 May 4. Int J Pediatr Otorhinolaryngol. 2013. PMID: 23648318 Free PMC article.
-
Mechanical amplification by hair cells in the semicircular canals.Proc Natl Acad Sci U S A. 2010 Feb 23;107(8):3864-9. doi: 10.1073/pnas.0906765107. Epub 2010 Feb 4. Proc Natl Acad Sci U S A. 2010. PMID: 20133682 Free PMC article.
-
Mechanotransduction by hair cells: models, molecules, and mechanisms.Cell. 2009 Oct 2;139(1):33-44. doi: 10.1016/j.cell.2009.09.010. Cell. 2009. PMID: 19804752 Free PMC article. Review.
-
Different rates of endocytic activity and vesicle transport from the apical and synaptic poles of the outer hair cell.HNO. 2019 Jun;67(6):449-457. doi: 10.1007/s00106-019-0674-y. HNO. 2019. PMID: 31073640 Free PMC article.
-
Biophysical mechanisms underlying outer hair cell loss associated with a shortened tectorial membrane.J Assoc Res Otolaryngol. 2011 Oct;12(5):577-94. doi: 10.1007/s10162-011-0269-0. Epub 2011 May 13. J Assoc Res Otolaryngol. 2011. PMID: 21567249 Free PMC article.
References
-
- Albert JT, Nadrowski B, Gopfert MC. Mechanical signatures of transducer gating in the Drosophila ear. Curr. Biol. 2007;17:1000–1006. - PubMed
-
- Allen JB, Fahey PF. Using acoustic distortion products to measure the cochlear amplifier gain on the basilar membrane. J. Acoust. Soc. Am. 1992;92:178–188. - PubMed
-
- Art JJ, Crawford AC, Fettiplace R, Fuchs PA. Efferent regulation of hair cells in the turtle cochlea. Proc. Roy. Soc. Lond B. 1982;216:377–384. - PubMed
-
- Ashmore J. Cochlear outer hair cell motility. Physiol. Rev. 2008;88:173–210. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

