Endothelial function and vascular oxidative stress in long-lived GH/IGF-deficient Ames dwarf mice
- PMID: 18757483
- PMCID: PMC2614588
- DOI: 10.1152/ajpheart.412.2008
Endothelial function and vascular oxidative stress in long-lived GH/IGF-deficient Ames dwarf mice
Abstract
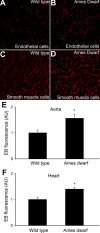
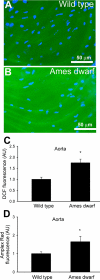
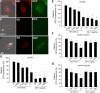
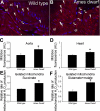
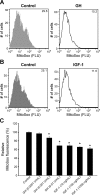
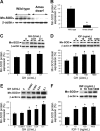
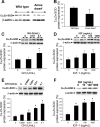
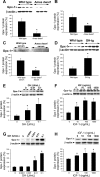
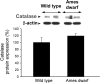
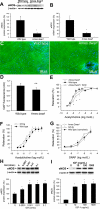
Hypopituitary Ames dwarf mice have low circulating growth hormone (GH)/IGF-I levels, and they have extended longevity and exhibit many symptoms of delayed aging. To elucidate the vascular consequences of Ames dwarfism we compared endothelial O2(-) and H2O2 production, mitochondrial reactive oxygen species (ROS) generation, expression of antioxidant enzymes, and nitric oxide (NO) production in aortas of Ames dwarf and wild-type control mice. In Ames dwarf aortas endothelial O2(-) and H2O2 production and ROS generation by mitochondria were enhanced compared with those in vessels of wild-type mice. In Ames dwarf aortas there was a less abundant expression of Mn-SOD, Cu,Zn-SOD, glutathione peroxidase (GPx)-1, and endothelial nitric oxide synthase (eNOS). NO production and acetylcholine-induced relaxation were also decreased in aortas of Ames dwarf mice. In cultured wild-type mouse aortas and in human coronary arterial endothelial cells treatment with GH and IGF significantly reduced cellular O2(-) and H2O2 production and ROS generation by mitochondria and upregulated expression of Mn-SOD, Cu,Zn-SOD, GPx-1, and eNOS. Thus GH and IGF-I promote antioxidant phenotypic changes in the endothelial cells, whereas Ames dwarfism leads to vascular oxidative stress.
Figures
Similar articles
-
Expression of oxidative phosphorylation components in mitochondria of long-living Ames dwarf mice.Age (Dordr). 2012 Feb;34(1):43-57. doi: 10.1007/s11357-011-9212-x. Epub 2011 Feb 16. Age (Dordr). 2012. PMID: 21327718 Free PMC article.
-
Vascular superoxide and hydrogen peroxide production and oxidative stress resistance in two closely related rodent species with disparate longevity.Aging Cell. 2007 Dec;6(6):783-97. doi: 10.1111/j.1474-9726.2007.00339.x. Epub 2007 Oct 8. Aging Cell. 2007. PMID: 17925005
-
Vasoprotective effects of life span-extending peripubertal GH replacement in Lewis dwarf rats.J Gerontol A Biol Sci Med Sci. 2010 Nov;65(11):1145-56. doi: 10.1093/gerona/glq147. Epub 2010 Aug 16. J Gerontol A Biol Sci Med Sci. 2010. PMID: 20713653 Free PMC article.
-
Life extension in the dwarf mouse.Curr Top Dev Biol. 2004;63:189-225. doi: 10.1016/S0070-2153(04)63006-7. Curr Top Dev Biol. 2004. PMID: 15536017 Review.
-
Functionally enhanced brown adipose tissue in Ames dwarf mice.Adipocyte. 2017 Jan 2;6(1):62-67. doi: 10.1080/21623945.2016.1274470. Epub 2016 Dec 21. Adipocyte. 2017. PMID: 28452585 Free PMC article. Review.
Cited by
-
Circulating factors induced by caloric restriction in the nonhuman primate Macaca mulatta activate angiogenic processes in endothelial cells.J Gerontol A Biol Sci Med Sci. 2013 Mar;68(3):235-49. doi: 10.1093/gerona/gls158. Epub 2012 Aug 17. J Gerontol A Biol Sci Med Sci. 2013. PMID: 22904098 Free PMC article.
-
Healthful aging mediated by inhibition of oxidative stress.Ageing Res Rev. 2020 Dec;64:101194. doi: 10.1016/j.arr.2020.101194. Epub 2020 Oct 19. Ageing Res Rev. 2020. PMID: 33091597 Free PMC article. Review.
-
Insulin-like growth factor 1 deficiency exacerbates hypertension-induced cerebral microhemorrhages in mice, mimicking the aging phenotype.Aging Cell. 2017 Jun;16(3):469-479. doi: 10.1111/acel.12583. Epub 2017 Mar 14. Aging Cell. 2017. PMID: 28295976 Free PMC article.
-
Comparing the Behavioural Effects of Exogenous Growth Hormone and Melatonin in Young and Old Wistar Rats.Oxid Med Cell Longev. 2016;2016:5863402. doi: 10.1155/2016/5863402. Epub 2016 Dec 5. Oxid Med Cell Longev. 2016. PMID: 28050228 Free PMC article.
-
Late Health Effects of Partial Body Irradiation Injury in a Minipig Model Are Associated with Changes in Systemic and Cardiac IGF-1 Signaling.Int J Mol Sci. 2021 Mar 23;22(6):3286. doi: 10.3390/ijms22063286. Int J Mol Sci. 2021. PMID: 33807089 Free PMC article.
References
-
- Al-Regaiey KA, Masternak MM, Bonkowski M, Sun L, Bartke A. Long-lived growth hormone receptor knockout mice: interaction of reduced insulin-like growth factor I/insulin signaling and caloric restriction. Endocrinology 146: 851–860, 2005. - PubMed
-
- Amador-Noguez D, Yagi K, Venable S, Darlington G. Gene expression profile of long-lived Ames dwarf mice and Little mice. Aging Cell 3: 423–441, 2004. - PubMed
-
- Bartke A, Brown-Borg H. Life extension in the dwarf mouse. Curr Top Dev Biol 63: 189–225, 2004. - PubMed
-
- Bartke A, Chandrashekar V, Bailey B, Zaczek D, Turyn D. Consequences of growth hormone (GH) overexpression and GH resistance. Neuropeptides 36: 201–208, 2002. - PubMed
-
- Boger RH, Skamira C, Bode-Boger SM, Brabant G, von zur Muhlen A, Frolich JC. Nitric oxide may mediate the hemodynamic effects of recombinant growth hormone in patients with acquired growth hormone deficiency. A double-blind, placebo-controlled study. J Clin Invest 98: 2706–2713, 1996. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

