PTEN-deficient cancers depend on PIK3CB
- PMID: 18755892
- PMCID: PMC2529105
- DOI: 10.1073/pnas.0802655105
PTEN-deficient cancers depend on PIK3CB
Abstract
Deregulation of the PI3K signaling pathway is observed in many human cancers and occurs most frequently through loss of PTEN phosphatase tumor suppressor function or through somatic activating mutations in the Class IA PI3K, PIK3CA. Tumors harboring activated p110alpha, the protein product of PIK3CA, require p110alpha activity for growth and survival and hence are expected to be responsive to inhibitors of its lipid kinase activity. Whether PTEN-deficient cancers similarly depend on p110alpha activity to sustain activation of the PI3K pathway has been unclear. In this study, we used a single-vector lentiviral inducible shRNA system to selectively inactivate the three Class IA PI3Ks, PIK3CA, PIK3CB, and PIK3CD, to determine which PI3K isoforms are responsible for driving the abnormal proliferation of PTEN-deficient cancers. Down-regulation of PIK3CA in colorectal cancer cells harboring mutations in PIK3CA inhibited downstream PI3K signaling and cell growth. Surprisingly, PIK3CA depletion affected neither PI3K signaling nor cell growth in 3 PTEN-deficient cancer cell lines. In contrast, down-regulation of the PIK3CB isoform, which encodes p110beta, resulted in pathway inactivation and subsequent inhibition of growth in both cell-based and in vivo settings. This essential function of PIK3CB in PTEN-deficient cancer cells required its lipid kinase activity. Our findings demonstrate that although p110alpha activation is required to sustain the proliferation of established PIK3CA-mutant tumors, PTEN-deficient tumors are dependent instead on p110beta signaling. This unexpected finding demonstrates the need to tailor therapeutic approaches to the genetic basis of PI3K pathway activation to achieve optimal treatment response.
Conflict of interest statement
Conflict of interest statement: All authors are employees of the Novartis Institutes for BioMedical Research.
Figures


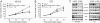
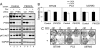
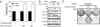
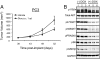
Similar articles
-
PI3K pathway dependencies in endometrioid endometrial cancer cell lines.Clin Cancer Res. 2013 Jul 1;19(13):3533-44. doi: 10.1158/1078-0432.CCR-12-3815. Epub 2013 May 14. Clin Cancer Res. 2013. PMID: 23674493 Free PMC article.
-
PI3K-p110α mediates resistance to HER2-targeted therapy in HER2+, PTEN-deficient breast cancers.Oncogene. 2016 Jul 7;35(27):3607-12. doi: 10.1038/onc.2015.406. Epub 2015 Oct 26. Oncogene. 2016. PMID: 26500061 Free PMC article.
-
Both p110α and p110β isoforms of PI3K can modulate the impact of loss-of-function of the PTEN tumour suppressor.Biochem J. 2012 Feb 15;442(1):151-9. doi: 10.1042/BJ20111741. Biochem J. 2012. PMID: 22150431 Free PMC article.
-
Class I Phosphoinositide 3-Kinase PIK3CA/p110α and PIK3CB/p110β Isoforms in Endometrial Cancer.Int J Mol Sci. 2018 Dec 7;19(12):3931. doi: 10.3390/ijms19123931. Int J Mol Sci. 2018. PMID: 30544563 Free PMC article. Review.
-
Should individual PI3 kinase isoforms be targeted in cancer?Curr Opin Cell Biol. 2009 Apr;21(2):199-208. doi: 10.1016/j.ceb.2008.12.007. Epub 2009 Feb 4. Curr Opin Cell Biol. 2009. PMID: 19200708 Free PMC article. Review.
Cited by
-
Activation of checkpoint kinase 2 is critical for herpes simplex virus type 1 replication in corneal epithelium.Ophthalmic Res. 2015;53(2):55-64. doi: 10.1159/000366228. Epub 2014 Dec 19. Ophthalmic Res. 2015. PMID: 25531207 Free PMC article.
-
Metabolic Imaging Detects Resistance to PI3Kα Inhibition Mediated by Persistent FOXM1 Expression in ER+ Breast Cancer.Cancer Cell. 2020 Oct 12;38(4):516-533.e9. doi: 10.1016/j.ccell.2020.08.016. Epub 2020 Sep 24. Cancer Cell. 2020. PMID: 32976773 Free PMC article.
-
Feedback suppression of PI3Kα signaling in PTEN-mutated tumors is relieved by selective inhibition of PI3Kβ.Cancer Cell. 2015 Jan 12;27(1):109-22. doi: 10.1016/j.ccell.2014.11.008. Epub 2014 Dec 24. Cancer Cell. 2015. PMID: 25544636 Free PMC article.
-
Dual inhibition of MEK and PI3Kβ/δ-a potential therapeutic strategy in PTEN-wild-type docetaxel-resistant metastatic prostate cancer.Front Pharmacol. 2024 Jan 22;15:1331648. doi: 10.3389/fphar.2024.1331648. eCollection 2024. Front Pharmacol. 2024. PMID: 38318136 Free PMC article.
-
PI3K pathway dependencies in endometrioid endometrial cancer cell lines.Clin Cancer Res. 2013 Jul 1;19(13):3533-44. doi: 10.1158/1078-0432.CCR-12-3815. Epub 2013 May 14. Clin Cancer Res. 2013. PMID: 23674493 Free PMC article.
References
-
- Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. - PubMed
-
- Salmena L, Carracedo A, Pandolfi PP. Tenets of PTEN tumor suppression. Cell. 2008;133:403–414. - PubMed
-
- Zhang J, Choi Y, Mavromatis B, Lichtenstein A, Li W. Preferential killing of PTEN-null myelomas by PI3K inhibitors through Akt pathway. Oncogene. 2003;22:6289–6295. - PubMed
-
- Bayascas JR, Leslie NR, Parsons R, Fleming S, Alessi DR. Hypomorphic mutation of PDK1 suppresses tumorigenesis in PTEN+/− mice. Curr Biol. 2005;15:1839–1846. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

