Erythropoietin-producing hepatocyte B6 variant-derived peptides with the ability to induce glioma-reactive cytotoxic T lymphocytes in human leukocyte antigen-A2+ glioma patients
- PMID: 18754880
- PMCID: PMC11158673
- DOI: 10.1111/j.1349-7006.2008.00866.x
Erythropoietin-producing hepatocyte B6 variant-derived peptides with the ability to induce glioma-reactive cytotoxic T lymphocytes in human leukocyte antigen-A2+ glioma patients
Abstract
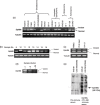
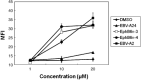
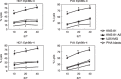
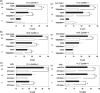
We recently cloned a variant form of erythropoietin-producing hepatocyte (Eph)B6, a member of the Eph receptor tyrosine kinase family. In the present study, we examined the expression of the EphB6 variant (EphB6v) in a panel of brain tumor cell lines and glioblastoma tissues and we found that EphB6v was preferentially expressed in malignant brain tumors, such as glioblastomas and anaplastic astrocytomas. The EphB6v has a unique 54 amino acid sequence at the C-terminal that is not found in normal EphB6. Therefore, we attempted to identify antigenic peptides unique to EphB6v for immunotherapy. The two EphB6v-derived peptides exhibited the ability to bind to human leukocyte antigen (HLA)-A0201 molecules, and each of them was able to induce cytotoxic T lymphocytes in vitro in the peripheral blood mononuclear cells of HLA-A2(+) glioma patients. The cytotoxicity was mediated by peptide-specific CD8(+) T cells in an HLA-A2-restricted manner. The expression of EphB6v was also observed in different types of cancer (e.g. lung, colon, stomach, liver and pancreatic) cells. Taken together, the two peptides derived from EphB6v might be appropriate targets for peptide-based specific immunotherapy for HLA-A2(+) patients with various cancers.
Figures

Similar articles
-
Identification of EphB6 variant-derived epitope peptides recognized by cytotoxic T-lymphocytes from HLA-A24+ malignant glioma patients.Oncol Rep. 2008 May;19(5):1277-83. Oncol Rep. 2008. PMID: 18425388
-
Identification of HLA-A2- and A24-restricted T-cell epitopes derived from SOX6 expressed in glioma stem cells for immunotherapy.Int J Cancer. 2010 Feb 15;126(4):919-29. doi: 10.1002/ijc.24851. Int J Cancer. 2010. PMID: 19728337
-
Kinesin superfamily protein-derived peptides with the ability to induce glioma-reactive cytotoxic T lymphocytes in human leukocyte antigen-A24+ glioma patients.Oncol Rep. 2007 Mar;17(3):629-36. Oncol Rep. 2007. PMID: 17273744
-
Identification of EGFRvIII-derived CTL epitopes restricted by HLA A0201 for dendritic cell based immunotherapy of gliomas.J Neurooncol. 2006 Jan;76(1):23-30. doi: 10.1007/s11060-005-3280-7. J Neurooncol. 2006. PMID: 16155724
-
Prostate-related antigen-derived new peptides having the capacity of inducing prostate cancer-reactive CTLs in HLA-A2+ prostate cancer patients.Oncol Rep. 2004 Sep;12(3):601-7. Oncol Rep. 2004. PMID: 15289844
Cited by
-
Ephs and Ephrins in malignant gliomas.Growth Factors. 2014 Dec;32(6):190-201. doi: 10.3109/08977194.2014.985787. Epub 2014 Nov 24. Growth Factors. 2014. PMID: 25418012 Free PMC article. Review.
-
Clinical relevance of Ephs and ephrins in cancer: lessons from breast, colorectal, and lung cancer profiling.Semin Cell Dev Biol. 2012 Feb;23(1):102-8. doi: 10.1016/j.semcdb.2011.10.014. Epub 2011 Oct 21. Semin Cell Dev Biol. 2012. PMID: 22040912 Free PMC article. Review.
-
Network-guided analysis of genes with altered somatic copy number and gene expression reveals pathways commonly perturbed in metastatic melanoma.PLoS One. 2011 Apr 8;6(4):e18369. doi: 10.1371/journal.pone.0018369. PLoS One. 2011. PMID: 21494657 Free PMC article.
-
Eph receptors and ephrins in cancer: bidirectional signalling and beyond.Nat Rev Cancer. 2010 Mar;10(3):165-80. doi: 10.1038/nrc2806. Nat Rev Cancer. 2010. PMID: 20179713 Free PMC article. Review.
-
Immunotherapeutic approaches for glioma.Crit Rev Immunol. 2009;29(1):1-42. doi: 10.1615/critrevimmunol.v29.i1.10. Crit Rev Immunol. 2009. PMID: 19348609 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

