Small functional groups for controlled differentiation of hydrogel-encapsulated human mesenchymal stem cells
- PMID: 18724374
- PMCID: PMC2929915
- DOI: 10.1038/nmat2269
Small functional groups for controlled differentiation of hydrogel-encapsulated human mesenchymal stem cells
Abstract
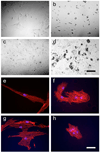
Cell-matrix interactions have critical roles in regeneration, development and disease. The work presented here demonstrates that encapsulated human mesenchymal stem cells (hMSCs) can be induced to differentiate down osteogenic and adipogenic pathways by controlling their three-dimensional environment using tethered small-molecule chemical functional groups. Hydrogels were formed using sufficiently low concentrations of tether molecules to maintain constant physical characteristics, encapsulation of hMSCs in three dimensions prevented changes in cell morphology, and hMSCs were shown to differentiate in normal growth media, indicating that the small-molecule functional groups induced differentiation. To our knowledge, this is the first example where synthetic matrices are shown to control induction of multiple hMSC lineages purely through interactions with small-molecule chemical functional groups tethered to the hydrogel material. Strategies using simple chemistry to control complex biological processes would be particularly powerful as they could make production of therapeutic materials simpler, cheaper and more easily controlled.
Figures



Similar articles
-
The performance of human mesenchymal stem cells encapsulated in cell-degradable polymer-peptide hydrogels.Biomaterials. 2011 May;32(14):3564-74. doi: 10.1016/j.biomaterials.2011.01.064. Epub 2011 Feb 21. Biomaterials. 2011. PMID: 21334063 Free PMC article.
-
In situ miRNA delivery from a hydrogel promotes osteogenesis of encapsulated mesenchymal stromal cells.Acta Biomater. 2020 Jan 1;101:249-261. doi: 10.1016/j.actbio.2019.11.016. Epub 2019 Nov 10. Acta Biomater. 2020. PMID: 31722255
-
The promotion of chondrogenesis, osteogenesis, and adipogenesis of human mesenchymal stem cells by multiple growth factors incorporated into nanosphere-coated microspheres.Biomaterials. 2011 Jan;32(1):28-38. doi: 10.1016/j.biomaterials.2010.08.088. Epub 2010 Sep 28. Biomaterials. 2011. PMID: 20875915
-
Attachment and spatial organisation of human mesenchymal stem cells on poly(ethylene glycol) hydrogels.J Mech Behav Biomed Mater. 2018 Aug;84:46-53. doi: 10.1016/j.jmbbm.2018.04.025. Epub 2018 May 3. J Mech Behav Biomed Mater. 2018. PMID: 29734041
-
Silk ionomers for encapsulation and differentiation of human MSCs.Biomaterials. 2012 Oct;33(30):7375-85. doi: 10.1016/j.biomaterials.2012.06.043. Epub 2012 Jul 21. Biomaterials. 2012. PMID: 22824008 Free PMC article.
Cited by
-
Regulation of the Stem Cell-Host Immune System Interplay Using Hydrogel Coencapsulation System with an Anti-Inflammatory Drug.Adv Funct Mater. 2015 Apr 15;25(15):2296-2307. doi: 10.1002/adfm.201500055. Adv Funct Mater. 2015. PMID: 26120294 Free PMC article.
-
Continuous gradient scaffolds for rapid screening of cell-material interactions and interfacial tissue regeneration.Acta Biomater. 2013 Sep;9(9):8254-61. doi: 10.1016/j.actbio.2013.05.012. Epub 2013 May 22. Acta Biomater. 2013. PMID: 23707502 Free PMC article.
-
Lubricated biodegradable polymer networks for regulating nerve cell behavior and fabricating nerve conduits with a compositional gradient.Biomacromolecules. 2012 Feb 13;13(2):358-68. doi: 10.1021/bm201372u. Epub 2012 Jan 18. Biomacromolecules. 2012. PMID: 22206477 Free PMC article.
-
Adipose-derived stem cell-secreted factors promote early stage follicle development in a biomimetic matrix.Biomater Sci. 2019 Jan 29;7(2):571-580. doi: 10.1039/c8bm01253a. Biomater Sci. 2019. PMID: 30608082 Free PMC article.
-
Nanotopography reveals metabolites that maintain the immunomodulatory phenotype of mesenchymal stromal cells.Nat Commun. 2023 Feb 10;14(1):753. doi: 10.1038/s41467-023-36293-7. Nat Commun. 2023. PMID: 36765065 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

