Identification of a copper-binding metallothionein in pathogenic mycobacteria
- PMID: 18724363
- PMCID: PMC2749609
- DOI: 10.1038/nchembio.109
Identification of a copper-binding metallothionein in pathogenic mycobacteria
Abstract
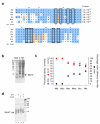
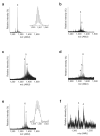
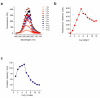
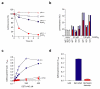
A screen of a genomic library from Mycobacterium tuberculosis (Mtb) identified a small, unannotated open reading frame (MT0196) that encodes a 4.9-kDa, cysteine-rich protein. Despite extensive nucleotide divergence, the amino acid sequence is highly conserved among mycobacteria that are pathogenic in vertebrate hosts. We synthesized the protein and found that it preferentially binds up to six Cu(I) ions in a solvent-shielded core. Copper, cadmium and compounds that generate nitric oxide or superoxide induced the gene's expression in Mtb up to 1,000-fold above normal expression. The native protein bound copper within Mtb and partially protected Mtb from copper toxicity. We propose that the product of the MT0196 gene be named mycobacterial metallothionein (MymT). To our knowledge, MymT is the first metallothionein of a Gram-positive bacterium with a demonstrated function.
Figures



Similar articles
-
A novel copper-responsive regulon in Mycobacterium tuberculosis.Mol Microbiol. 2011 Jan;79(1):133-48. doi: 10.1111/j.1365-2958.2010.07431.x. Epub 2010 Oct 29. Mol Microbiol. 2011. PMID: 21166899 Free PMC article.
-
A copper-induced metallothionein gene from Exopalaemon carinicauda and its response to heavy metal ions.Int J Biol Macromol. 2014 Sep;70:246-50. doi: 10.1016/j.ijbiomac.2014.06.020. Epub 2014 Jun 24. Int J Biol Macromol. 2014. PMID: 24971556
-
Cloning and sequencing of cDNAs encoding for a novel copper-specific metallothionein and two cadmium-inducible metallothioneins from the blue crab Callinectes sapidus.Comp Biochem Physiol C Toxicol Pharmacol. 2000 Mar;125(3):325-32. doi: 10.1016/s0742-8413(99)00114-0. Comp Biochem Physiol C Toxicol Pharmacol. 2000. PMID: 11790353
-
Regulation of metallothionein gene expression.Prog Food Nutr Sci. 1990;14(2-3):193-258. Prog Food Nutr Sci. 1990. PMID: 2293243 Review.
-
Bacterial metallothioneins: past, present, and questions for the future.J Biol Inorg Chem. 2011 Oct;16(7):1011-24. doi: 10.1007/s00775-011-0790-y. Epub 2011 May 19. J Biol Inorg Chem. 2011. PMID: 21594652 Review.
Cited by
-
Proteomic Analysis of Drug-Resistant Mycobacteria: Co-Evolution of Copper and INH Resistance.PLoS One. 2015 Jun 2;10(6):e0127788. doi: 10.1371/journal.pone.0127788. eCollection 2015. PLoS One. 2015. PMID: 26035302 Free PMC article.
-
A Role for Mycobacterium tuberculosis Sigma Factor C in Copper Nutritional Immunity.Int J Mol Sci. 2021 Feb 20;22(4):2118. doi: 10.3390/ijms22042118. Int J Mol Sci. 2021. PMID: 33672733 Free PMC article.
-
Nutritional immunity: the impact of metals on lung immune cells and the airway microbiome during chronic respiratory disease.Respir Res. 2021 Apr 29;22(1):133. doi: 10.1186/s12931-021-01722-y. Respir Res. 2021. PMID: 33926483 Free PMC article. Review.
-
Copper in microbial pathogenesis: meddling with the metal.Cell Host Microbe. 2012 Feb 16;11(2):106-15. doi: 10.1016/j.chom.2012.01.009. Cell Host Microbe. 2012. PMID: 22341460 Free PMC article. Review.
-
Screening we can believe in.Nat Chem Biol. 2009 Mar;5(3):127. doi: 10.1038/nchembio0309-127. Nat Chem Biol. 2009. PMID: 19219008
References
-
- Cole ST, et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393:537–44. - PubMed
-
- Gold B, Rodriguez GM, Marras SA, Pentecost M, Smith I. The Mycobacterium tuberculosis IdeR is a dual functional regulator that controls transcription of genes involved in iron acquisition, iron storage and survival in macrophages. Mol Microbiol. 2001;42:851–65. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

