A dosage-dependent requirement for Sox9 in pancreatic endocrine cell formation
- PMID: 18723011
- PMCID: PMC2879081
- DOI: 10.1016/j.ydbio.2008.07.034
A dosage-dependent requirement for Sox9 in pancreatic endocrine cell formation
Abstract
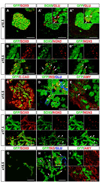
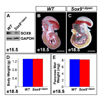
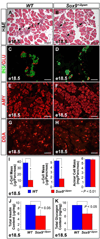
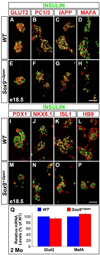
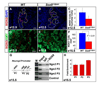
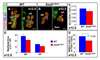
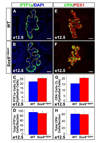
We have previously shown the transcription factor SOX9 to be required for the maintenance of multipotential pancreatic progenitor cells in the early embryonic pancreas. However, the association of pancreatic endocrine defects with the Sox9-haploinsufficiency syndrome campomelic dysplasia (CD) implies additional later roles for Sox9 in endocrine development. Using short-term lineage tracing in mice, we demonstrate here that SOX9 marks a pool of multipotential pancreatic progenitors throughout the window of major cell differentiation. During mid-pancreogenesis, both endocrine and exocrine cells simultaneously arise from the SOX9(+) epithelial cords. Our analysis of mice with 50%-reduced Sox9 gene dosage in pancreatic progenitors reveals endocrine-specific defects phenocopying CD. By birth, these mice display a specific reduction in endocrine cell mass, while their exocrine compartment and total organ size is normal. The decrease in endocrine cells is caused by reduced generation of endocrine progenitors from the SOX9(+) epithelium. Conversely, formation of exocrine progenitors is insensitive to reduced Sox9 gene dosage, thus explaining the normal organ size at birth. Our results show that not only is SOX9 required for the maintenance of early pancreatic progenitors, but also governs their adoption of an endocrine fate. Our findings therefore suggest that defective endocrine specification might underlie the pancreatic phenotype of individuals with CD.
Figures
Similar articles
-
Sox9+ ductal cells are multipotent progenitors throughout development but do not produce new endocrine cells in the normal or injured adult pancreas.Development. 2011 Feb;138(4):653-65. doi: 10.1242/dev.056499. Development. 2011. PMID: 21266405 Free PMC article.
-
Sox9: a master regulator of the pancreatic program.Rev Diabet Stud. 2014 Spring;11(1):51-83. doi: 10.1900/RDS.2014.11.51. Epub 2014 May 10. Rev Diabet Stud. 2014. PMID: 25148367 Free PMC article. Review.
-
Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine.Nat Genet. 2011 Jan;43(1):34-41. doi: 10.1038/ng.722. Epub 2010 Nov 28. Nat Genet. 2011. PMID: 21113154
-
A Sox9/Fgf feed-forward loop maintains pancreatic organ identity.Development. 2012 Sep;139(18):3363-72. doi: 10.1242/dev.078733. Epub 2012 Aug 8. Development. 2012. PMID: 22874919 Free PMC article.
-
The role of SOX9 transcription factor in pancreatic and duodenal development.Stem Cells Dev. 2013 Nov 15;22(22):2935-43. doi: 10.1089/scd.2013.0106. Epub 2013 Aug 2. Stem Cells Dev. 2013. PMID: 23806070 Review.
Cited by
-
Stem cell therapy: a potential for the perils of pancreatitis.Turk J Gastroenterol. 2020 Jun;31(6):415-424. doi: 10.5152/tjg.2020.19143. Turk J Gastroenterol. 2020. PMID: 32721912 Free PMC article. Review.
-
SOX9 Triggers Different Epithelial to Mesenchymal Transition States to Promote Pancreatic Cancer Progression.Cancers (Basel). 2022 Feb 12;14(4):916. doi: 10.3390/cancers14040916. Cancers (Basel). 2022. PMID: 35205666 Free PMC article.
-
Sox9+ ductal cells are multipotent progenitors throughout development but do not produce new endocrine cells in the normal or injured adult pancreas.Development. 2011 Feb;138(4):653-65. doi: 10.1242/dev.056499. Development. 2011. PMID: 21266405 Free PMC article.
-
Generation of a Quantitative Luciferase Reporter for Sox9 SUMOylation.Int J Mol Sci. 2020 Feb 13;21(4):1274. doi: 10.3390/ijms21041274. Int J Mol Sci. 2020. PMID: 32070068 Free PMC article.
-
Feedback control of growth, differentiation, and morphogenesis of pancreatic endocrine progenitors in an epithelial plexus niche.Genes Dev. 2015 Oct 15;29(20):2203-16. doi: 10.1101/gad.267914.115. Genes Dev. 2015. PMID: 26494792 Free PMC article.
References
-
- Akiyama H, et al. The transcription factor Sox9 is degraded by the ubiquitin-proteasome system and stabilized by a mutation in a ubiquitintarget site. Matrix Biol. 2005a;23:499–505. - PubMed
-
- Barrionuevo F, et al. Homozygous inactivation of Sox9 causes complete XY sex reversal in mice. Biol Reprod. 2006;74:195–201. - PubMed
-
- Bernard P, et al. Dimerization of SOX9 is required for chondrogenesis, but not for sex determination. Hum Mol Genet. 2003;12:1755–1765. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

