Opioid receptors: from binding sites to visible molecules in vivo
- PMID: 18718480
- PMCID: PMC2950281
- DOI: 10.1016/j.neuropharm.2008.07.033
Opioid receptors: from binding sites to visible molecules in vivo
Abstract
Opioid drugs such as heroin interact directly with opioid receptors whilst other addictive drugs, including marijuana, alcohol and nicotine indirectly activate endogenous opioid systems to contribute to their rewarding properties. The opioid system therefore plays a key role in addiction neurobiology and continues to be a primary focus for NIDA-supported research. Opioid receptors and their peptide ligands, the endorphins and enkephalins, form an extensive heterogeneous network throughout the central and peripheral nervous system. In addition to reward, opioid drugs regulate many functions such that opioid receptors are targets of choice in several physiological, neurological and psychiatric disorders. Because of the multiplicity and diversity of ligands and receptors, opioid receptors have served as an optimal model for G protein coupled receptor (GPCR) research. The isolation of opioid receptor genes opened the way to molecular manipulations of the receptors, both in artificial systems and in vivo, contributing to our current understanding of the diversity of opioid receptor biology at the behavioral, cellular and molecular levels. This review will briefly summarize some aspects of current knowledge that has accumulated since the very early characterization of opioid receptor genes. Importantly, we will identify a number of research directions that are likely to develop during the next decade.
Figures
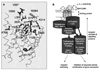
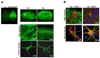

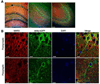
Similar articles
-
15 years of genetic approaches in vivo for addiction research: Opioid receptor and peptide gene knockout in mouse models of drug abuse.Neuropharmacology. 2014 Jan;76 Pt B(0 0):204-17. doi: 10.1016/j.neuropharm.2013.08.028. Epub 2013 Sep 10. Neuropharmacology. 2014. PMID: 24035914 Free PMC article. Review.
-
The opioid receptors as targets for drug abuse medication.Br J Pharmacol. 2015 Aug;172(16):3964-79. doi: 10.1111/bph.13190. Epub 2015 Jun 26. Br J Pharmacol. 2015. PMID: 25988826 Free PMC article. Review.
-
Reward processing by the opioid system in the brain.Physiol Rev. 2009 Oct;89(4):1379-412. doi: 10.1152/physrev.00005.2009. Physiol Rev. 2009. PMID: 19789384 Free PMC article. Review.
-
Evolving perspectives on neurobiological research on the addictions: celebration of the 30th anniversary of NIDA.Neuropharmacology. 2004;47 Suppl 1:324-44. doi: 10.1016/j.neuropharm.2004.07.024. Neuropharmacology. 2004. PMID: 15464148 Review.
-
Endogenous opioid systems and alcohol addiction.Psychopharmacology (Berl). 1997 Jan;129(2):99-111. doi: 10.1007/s002130050169. Psychopharmacology (Berl). 1997. PMID: 9040115 Review.
Cited by
-
Candidate Biomarkers of Suicide Crisis Syndrome: What to Test Next? A Concept Paper.Int J Neuropsychopharmacol. 2020 Apr 21;23(3):192-205. doi: 10.1093/ijnp/pyz063. Int J Neuropsychopharmacol. 2020. PMID: 31781761 Free PMC article. Review.
-
Mouse δ opioid receptors are located on presynaptic afferents to hippocampal pyramidal cells.Cell Mol Neurobiol. 2012 May;32(4):509-16. doi: 10.1007/s10571-011-9791-1. Epub 2012 Jan 18. Cell Mol Neurobiol. 2012. PMID: 22252784 Free PMC article.
-
Heteromerization Modulates mu Opioid Receptor Functional Properties in vivo.Front Pharmacol. 2018 Nov 13;9:1240. doi: 10.3389/fphar.2018.01240. eCollection 2018. Front Pharmacol. 2018. PMID: 30483121 Free PMC article. Review.
-
Strain and sex-related behavioral variability of oxycodone dependence in rats.Neuropharmacology. 2023 Oct 1;237:109635. doi: 10.1016/j.neuropharm.2023.109635. Epub 2023 Jun 15. Neuropharmacology. 2023. PMID: 37327971 Free PMC article.
-
Neuropeptides as mediators of the early-life impact on the brain; implications for alcohol use disorders.Front Mol Neurosci. 2012 Jul 5;5:77. doi: 10.3389/fnmol.2012.00077. eCollection 2012. Front Mol Neurosci. 2012. PMID: 22783165 Free PMC article.
References
-
- Akil H, Watson SJ, Young E, Lewis ME, Khachaturian H, Walker JJ. Endogenous opioids: Biology and function. Ann. Rev. Neurosci. 1984;7:223–255. - PubMed
-
- Ammon S, Mayer P, Riechert U, Tischmeyer H, Hollt V. Microarray analysis of genes expressed in the frontal cortex of rats chronically treated with morphine and after naloxone precipitated withdrawal. Brain Res Mol Brain Res. 2003;112:113–125. - PubMed
-
- Arden JR, Segredo V, Wang Z, Lameh J, Sadée W. Phosphorylation and agonist specific intracellular trafficking of an epitope-tagged m-opioid receptor expressed in HEK 293 cells. J. Neurochem. 1995;65:1636–1645. - PubMed
-
- Baler RD, Volkow ND. Drug addiction: the neurobiology of disrupted self-control. Trends Mol Med. 2006;12:559–566. - PubMed
-
- Befort K, Filliol D, Darcq E, Ghate A, Matifas A, Lardenois A, Muller J, Thibault C, Dembele D, Poch O, Kieffer BL. Gene altered expression in lateral hypothalamus upon mu opioid receptor activation by morphine Annals of the New York Academy of Sciences. in press. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

