Activation-induced cytidine deaminase deficiency causes organ-specific autoimmune disease
- PMID: 18716662
- PMCID: PMC2515643
- DOI: 10.1371/journal.pone.0003033
Activation-induced cytidine deaminase deficiency causes organ-specific autoimmune disease
Abstract
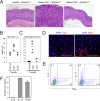
Activation-induced cytidine deaminase (AID) expressed by germinal center B cells is a central regulator of somatic hypermutation (SHM) and class switch recombination (CSR). Humans with AID mutations develop not only the autosomal recessive form of hyper-IgM syndrome (HIGM2) associated with B cell hyperplasia, but also autoimmune disorders by unknown mechanisms. We report here that AID-/- mice spontaneously develop tertiary lymphoid organs (TLOs) in non-lymphoid tissues including the stomach at around 6 months of age. At a later stage, AID-/- mice develop a severe gastritis characterized by loss of gastric glands and epithelial hyperplasia. The disease development was not attenuated even under germ-free (GF) conditions. Gastric autoantigen -specific serum IgM was elevated in AID-/- mice, and the serum levels correlated with the gastritis pathological score. Adoptive transfer experiments suggest that autoimmune CD4+ T cells mediate gastritis development as terminal effector cells. These results suggest that abnormal B-cell expansion due to AID deficiency can drive B-cell autoimmunity, and in turn promote TLO formation, which ultimately leads to the propagation of organ-specific autoimmune effector CD4+ T cells. Thus, AID plays an important role in the containment of autoimmune diseases by negative regulation of autoreactive B cells.
Conflict of interest statement
Figures




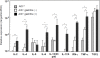


Similar articles
-
Activation-induced cytidine deaminase (AID) deficiency causes the autosomal recessive form of the Hyper-IgM syndrome (HIGM2).Cell. 2000 Sep 1;102(5):565-75. doi: 10.1016/s0092-8674(00)00079-9. Cell. 2000. PMID: 11007475
-
Activation-induced cytidine deaminase expression in follicular dendritic cell networks and interfollicular large B cells supports functionality of ectopic lymphoid neogenesis in autoimmune sialoadenitis and MALT lymphoma in Sjögren's syndrome.J Immunol. 2007 Oct 1;179(7):4929-38. doi: 10.4049/jimmunol.179.7.4929. J Immunol. 2007. PMID: 17878393
-
A Novel AICDA Splice-Site Mutation in Two Siblings with HIGM2 Permits Somatic Hypermutation but Abrogates Mutational Targeting.J Clin Immunol. 2022 May;42(4):771-782. doi: 10.1007/s10875-022-01233-5. Epub 2022 Mar 5. J Clin Immunol. 2022. PMID: 35246784 Free PMC article.
-
Potential roles of activation-induced cytidine deaminase in promotion or prevention of autoimmunity in humans.Autoimmunity. 2013 Mar;46(2):148-56. doi: 10.3109/08916934.2012.750299. Epub 2013 Jan 10. Autoimmunity. 2013. PMID: 23215867 Free PMC article. Review.
-
Activation-induced cytidine deaminase: a dual role in class-switch recombination and somatic hypermutation.Eur J Immunol. 2003 Aug;33(8):2069-73. doi: 10.1002/eji.200324133. Eur J Immunol. 2003. PMID: 12884279 Review.
Cited by
-
Deficiency in activation-induced cytidine deaminase promotes systemic autoimmunity in lpr mice on a C57BL/6 background.Clin Exp Immunol. 2010 Feb;159(2):169-75. doi: 10.1111/j.1365-2249.2009.04058.x. Epub 2009 Nov 18. Clin Exp Immunol. 2010. PMID: 19922498 Free PMC article.
-
Quorum sensing contributes to activated IgM-secreting B cell homeostasis.J Immunol. 2013 Jan 1;190(1):106-14. doi: 10.4049/jimmunol.1200907. Epub 2012 Dec 3. J Immunol. 2013. PMID: 23209322 Free PMC article.
-
Self-Antigen-Driven Thymic B Cell Class Switching Promotes T Cell Central Tolerance.Cell Rep. 2016 Oct 4;17(2):387-398. doi: 10.1016/j.celrep.2016.09.011. Cell Rep. 2016. PMID: 27705788 Free PMC article.
-
The role of gut microbiota in immune homeostasis and autoimmunity.Gut Microbes. 2012 Jan-Feb;3(1):4-14. doi: 10.4161/gmic.19320. Epub 2012 Jan 1. Gut Microbes. 2012. PMID: 22356853 Free PMC article. Review.
-
Functions and Malfunctions of Mammalian DNA-Cytosine Deaminases.Chem Rev. 2016 Oct 26;116(20):12688-12710. doi: 10.1021/acs.chemrev.6b00296. Epub 2016 Sep 1. Chem Rev. 2016. PMID: 27585283 Free PMC article. Review.
References
-
- Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, et al. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000;102:553–563. - PubMed
-
- Revy P, Muto T, Levy Y, Geissmann F, Plebani A, et al. Activation-induced cytidine deaminase (AID) deficiency causes the autosomal recessive form of the Hyper-IgM syndrome (HIGM2). Cell. 2000;102:565–575. - PubMed
-
- Fagarasan S, Muramatsu M, Suzuki K, Nagaoka H, Hiai H, et al. Critical roles of activation-induced cytidine deaminase in the homeostasis of gut flora. Science. 2002;298:1424–1427. - PubMed
-
- Quartier P, Bustamante J, Sanal O, Plebani A, Debre M, et al. Clinical, immunologic and genetic analysis of 29 patients with autosomal recessive hyper-IgM syndrome due to Activation-Induced Cytidine Deaminase deficiency. Clin Immunol. 2004;110:22–29. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

