Erk5 controls Slug expression and keratinocyte activation during wound healing
- PMID: 18716062
- PMCID: PMC2575153
- DOI: 10.1091/mbc.e07-10-1078
Erk5 controls Slug expression and keratinocyte activation during wound healing
Abstract
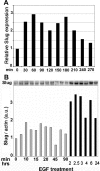
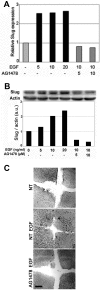
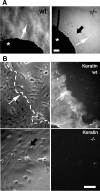
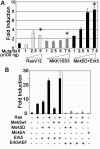
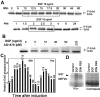
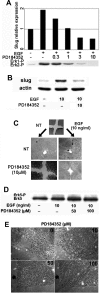
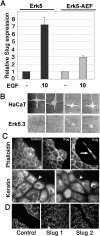
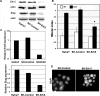
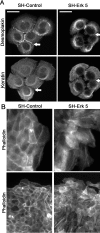
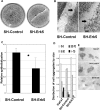
Reepithelialization during cutaneous wound healing involves numerous signals that result in basal keratinocyte activation, spreading, and migration, all linked to a loosening of cell-cell adhesion structures. The transcription factor Slug is required for this process, and EGF treatment of human keratinocytes induced activating phosphorylation of Erk5 that coincides with slug transcription. Accordingly, ectopic activation of Erk5 led to increased Slug mRNA levels and faster wound healing, whereas keratinocyte migration was totally blocked by Erk5 pathway inhibition. Expression of a shRNA specific for Erk5 strongly diminished Erk5 levels in keratinocytes and significantly decreased their motility response to EGF, along with induction of Slug expression. These Erk5-deprived keratinocytes showed an altered, more compact morphology, along with disruption of desmosome organization. Accordingly, they displayed an altered ability to form cell aggregates. These results implicate a novel EGFR/Erk5/Slug pathway in the control of cytoskeleton organization and cell motility in keratinocytes treated with EGF.
Figures
Similar articles
-
Slug/Snai2 is a downstream mediator of epidermal growth factor receptor-stimulated reepithelialization.J Invest Dermatol. 2009 Feb;129(2):491-5. doi: 10.1038/jid.2008.222. Epub 2008 Aug 14. J Invest Dermatol. 2009. PMID: 18685621 Free PMC article.
-
ERK1/2-driven and MKP-mediated inhibition of EGF-induced ERK5 signaling in human proximal tubular cells.J Cell Physiol. 2007 Apr;211(1):88-100. doi: 10.1002/jcp.20909. J Cell Physiol. 2007. PMID: 17131384
-
Activation of either ERK1/2 or ERK5 MAP kinase pathways can lead to disruption of the actin cytoskeleton.J Cell Sci. 2005 Apr 15;118(Pt 8):1663-71. doi: 10.1242/jcs.02308. Epub 2005 Mar 29. J Cell Sci. 2005. PMID: 15797923
-
Developmental transcription factor slug is required for effective re-epithelialization by adult keratinocytes.J Cell Physiol. 2005 Mar;202(3):858-66. doi: 10.1002/jcp.20188. J Cell Physiol. 2005. PMID: 15389643
-
The skinny on Slug.Mol Carcinog. 2010 Oct;49(10):851-61. doi: 10.1002/mc.20674. Mol Carcinog. 2010. PMID: 20721976 Free PMC article. Review.
Cited by
-
Involvement of Epithelial-Mesenchymal Transition (EMT) in Autoimmune Diseases.Int J Mol Sci. 2023 Sep 23;24(19):14481. doi: 10.3390/ijms241914481. Int J Mol Sci. 2023. PMID: 37833928 Free PMC article. Review.
-
Identification of biomechanical force as a novel inducer of epithelial-mesenchymal transition features in mechanical stretched skin.Am J Transl Res. 2015 Nov 15;7(11):2187-98. eCollection 2015. Am J Transl Res. 2015. PMID: 26807167 Free PMC article.
-
Cooperation and Interplay between EGFR Signalling and Extracellular Vesicle Biogenesis in Cancer.Cells. 2020 Dec 8;9(12):2639. doi: 10.3390/cells9122639. Cells. 2020. PMID: 33302515 Free PMC article. Review.
-
Slug controls stem/progenitor cell growth dynamics during mammary gland morphogenesis.PLoS One. 2012;7(12):e53498. doi: 10.1371/journal.pone.0053498. Epub 2012 Dec 27. PLoS One. 2012. PMID: 23300933 Free PMC article.
-
The translational significance of epithelial-mesenchymal transition in head and neck cancer.Clin Transl Med. 2014 Nov 30;3(1):60. doi: 10.1186/s40169-014-0039-9. eCollection 2014 Dec. Clin Transl Med. 2014. PMID: 25632320 Free PMC article.
References
-
- Arnoux V., Come C., Kusewitt D., Savagner P. Cutaneous wound healing: a partial and reversible EMT. In: Savagner P., editor. Rise and Fall of Epithelial Phenotype: Concepts of Epithelial-Mesenchymal Transition. Austin, TX: Landes Biosciences; 2005. pp. 111–134.
-
- Barros J. C., Marshall C. J. Activation of either ERK1/2 or ERK5 MAP kinase pathways can lead to disruption of the actin cytoskeleton. J. Cell Sci. 2005;118:1663–1671. - PubMed
-
- Boukamp P., Chen J., Gonzales F., Jones P. A., Fusenig N. E. Progressive stages of “transdifferentiation” from epidermal to mesenchymal phenotype induced by MyoD1 transfection, 5-aza-2′-deoxycytidine treatment, and selection for reduced cell attachment in the human keratinocyte line HaCaT. J. Cell Biol. 1992;116:1257–1271. - PMC - PubMed
-
- Brown G. L., Nanney L. B., Griffen J., Cramer A. B., Yancey J. M., Curtsinger L. J., 3rd, Holtzin L., Schultz G. S., Jurkiewicz M. J., Lynch J. B. Enhancement of wound healing by topical treatment with epidermal growth factor. N. Engl. J. Med. 1989;321:76–79. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

