Identification and characterization of infiltrating macrophages in acetaminophen-induced liver injury
- PMID: 18713872
- PMCID: PMC2614594
- DOI: 10.1189/jlb.0308173
Identification and characterization of infiltrating macrophages in acetaminophen-induced liver injury
Abstract
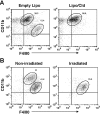
The role of macrophages in the pathogenesis of acetaminophen (APAP)-induced liver injury remains controversial, as it has been demonstrated that these cells display pro-toxicant and hepato-protective functions. This controversy may stem from the heterogeneity and/or plasticity of macrophages and the difficulty in distinguishing and differentially studying subpopulations of macrophages in the liver. In the present study, using flow cytometric analysis and fluorescence-labeled antibodies against specific cell surface macrophage markers, we were able to, for the first time, identify an APAP-induced macrophage (IM) population distinct from resident Kupffer cells. The data demonstrated that the IMs were derived from circulating monocytes that infiltrated the liver following APAP-induced liver injury. The IMs exhibited a phenotype consistent with that of alternatively activated macrophages and demonstrated the ability to phagocytize apoptotic cells and induce apoptosis of neutrophils. Furthermore, in the absence of the IMs, the resolution of hepatic damage following APAP-induced hepatotoxicity was delayed in CCR2(-/-) mice compared with wild-type mice. These findings likely contribute to the role of the IMs in the processes of tissue repair, including counteracting inflammation and promoting angiogenesis. The present study also demonstrated the ability of separating populations of macrophages and delineating distinct functions of each group in future studies of inflammatory disease in the liver and other tissues.
Figures



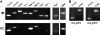
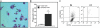

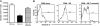

Similar articles
-
Chemokine (C-C motif) receptor 2-positive monocytes aggravate the early phase of acetaminophen-induced acute liver injury.Hepatology. 2016 Nov;64(5):1667-1682. doi: 10.1002/hep.28682. Epub 2016 Jul 22. Hepatology. 2016. PMID: 27302828
-
Role of CCR2 in macrophage migration into the liver during acetaminophen-induced hepatotoxicity in the mouse.Hepatology. 2002 May;35(5):1093-103. doi: 10.1053/jhep.2002.33162. Hepatology. 2002. PMID: 11981759
-
Role of hepatic resident and infiltrating macrophages in liver repair after acute injury.Biochem Pharmacol. 2013 Sep 15;86(6):836-43. doi: 10.1016/j.bcp.2013.07.006. Epub 2013 Jul 19. Biochem Pharmacol. 2013. PMID: 23876342 Free PMC article.
-
Autophagy and acetaminophen-induced hepatotoxicity.Arch Toxicol. 2018 Jul;92(7):2153-2161. doi: 10.1007/s00204-018-2237-5. Epub 2018 Jun 6. Arch Toxicol. 2018. PMID: 29876591 Review.
-
Macrophages in necrotic liver lesion repair: opportunities for therapeutical applications.Am J Physiol Cell Physiol. 2024 May 1;326(5):C1556-C1562. doi: 10.1152/ajpcell.00053.2024. Epub 2024 Apr 15. Am J Physiol Cell Physiol. 2024. PMID: 38618702 Review.
Cited by
-
Models of drug-induced liver injury for evaluation of phytotherapeutics and other natural products.Food Chem Toxicol. 2013 May;55:279-89. doi: 10.1016/j.fct.2012.12.063. Epub 2013 Jan 22. Food Chem Toxicol. 2013. PMID: 23353004 Free PMC article. Review.
-
Regulation of innate responses during pre-patent schistosome infection provides an immune environment permissive for parasite development.PLoS Pathog. 2013;9(10):e1003708. doi: 10.1371/journal.ppat.1003708. Epub 2013 Oct 10. PLoS Pathog. 2013. PMID: 24130499 Free PMC article.
-
Inflammatory responses are not sufficient to cause delayed neuronal death in ATP-induced acute brain injury.PLoS One. 2010 Oct 29;5(10):e13756. doi: 10.1371/journal.pone.0013756. PLoS One. 2010. PMID: 21060796 Free PMC article.
-
Alternatively activated macrophages promote resolution of necrosis following acute liver injury.J Hepatol. 2020 Aug;73(2):349-360. doi: 10.1016/j.jhep.2020.02.031. Epub 2020 Mar 11. J Hepatol. 2020. PMID: 32169610 Free PMC article.
-
The effect of liver disease on hepatic microenvironment and implications for immune therapy.Front Pharmacol. 2023 Aug 7;14:1225821. doi: 10.3389/fphar.2023.1225821. eCollection 2023. Front Pharmacol. 2023. PMID: 37608898 Free PMC article. Review.
References
-
- Hartleb M, Biernat L, Kochel A. Drug-induced liver damage—a three-year study of patients from one gastroenterological department. Med Sci Monit. 2002;8:CR292–CR296. - PubMed
-
- Lazarou J, Pomeranz B H, Corey P N. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA. 1998;279:1200–1205. - PubMed
-
- Lazerow S K, Abdi M S, Lewis J H. Drug-induced liver disease 2004. Curr Opin Gastroenterol. 2005;21:283–292. - PubMed
-
- Lee W M, Senior J R. Recognizing drug-induced liver injury: current problems, possible solutions. Toxicol Pathol. 2005;33:155–164. - PubMed
-
- Lee W M. Drug-induced hepatotoxicity. N Engl J Med. 2003;349:474–485. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

