Reinduction platform for children with first marrow relapse of acute lymphoblastic Leukemia: A Children's Oncology Group Study[corrected]
- PMID: 18711187
- PMCID: PMC2654313
- DOI: 10.1200/JCO.2008.16.1414
Reinduction platform for children with first marrow relapse of acute lymphoblastic Leukemia: A Children's Oncology Group Study[corrected]
Erratum in
- J Clin Oncol. 2008 Oct 1;26(28): 4697.
Abstract
Purpose: Treatment of childhood relapsed acute lymphoblastic leukemia (ALL) remains a significant challenge. The goal of the Children's Oncology Group (COG) AALL01P2 study was to develop a safe and active chemotherapy reinduction platform, which could be used to evaluate novel agents in future trials.
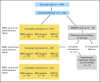
Patients and methods: One hundred twenty-four patients with ALL and first marrow relapse received three, 35-day blocks of reinduction chemotherapy: 69 with early relapse (ER; < 36 months from initial diagnosis) and 55 with late relapse (LR). Minimal residual disease (MRD) was measured by flow cytometry after each treatment block.
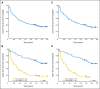
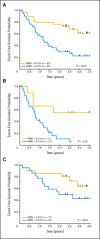
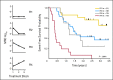
Results: Second complete remission (CR2) rates at the end of block 1 in 117 assessable patients were 68% +/- 6% for ER (n = 63) and 96% +/- 3% for LR (n = 54; P < .0001). Five of seven patients with T-cell ALL (T-ALL) failed to achieve CR2. Among patients in CR2, MRD greater than 0.01% was detected at the end of block 1 in 75% +/- 7% of ER (n = 36) versus 51% +/- 8% of LR (n = 43; P = .0375) and 12-month event-free survival was 80% +/- 7% versus 58% +/- 7% in MRD-negative versus positive patients (P < .0005). Blocks 2 and 3 of therapy resulted in reduction of MRD burden in 40 of 56 patients who were MRD positive after block 1. Toxicity was acceptable during all three blocks with five deaths (4%) from infections.
Conclusion: The AALL01P2 regimen is a tolerable and active reinduction platform, suitable for testing in combination with novel agents in B-precursor ALL. Alternative strategies are needed for T-ALL. Serial MRD measurements were feasible and prognostic of outcome.
Figures
Similar articles
-
Toxicity and efficacy of intensive chemotherapy for children with acute lymphoblastic leukemia (ALL) after first bone marrow or extramedullary relapse.Pediatr Blood Cancer. 2004 Oct;43(5):571-9. doi: 10.1002/pbc.20128. Pediatr Blood Cancer. 2004. PMID: 15382275 Clinical Trial.
-
Phase I trial of the mTOR inhibitor everolimus in combination with multi-agent chemotherapy in relapsed childhood acute lymphoblastic leukemia.Pediatr Blood Cancer. 2018 Jul;65(7):e27062. doi: 10.1002/pbc.27062. Epub 2018 Mar 30. Pediatr Blood Cancer. 2018. PMID: 29603593 Clinical Trial.
-
Novel multiagent chemotherapy for bone marrow relapse of pediatric acute lymphoblastic leukemia.Med Pediatr Oncol. 2000 May;34(5):313-8. doi: 10.1002/(sici)1096-911x(200005)34:5<313::aid-mpo1>3.0.co;2-q. Med Pediatr Oncol. 2000. PMID: 10797352 Clinical Trial.
-
Low relapse rate in children with acute lymphoblastic leukemia after risk-directed therapy.J Pediatr Hematol Oncol. 2001 Dec;23(9):591-7. doi: 10.1097/00043426-200112000-00008. J Pediatr Hematol Oncol. 2001. PMID: 11902303 Review.
-
Minimal Residual Disease Evaluation in Childhood Acute Lymphoblastic Leukemia: An Economic Analysis.Ont Health Technol Assess Ser. 2016 Mar 8;16(8):1-83. eCollection 2016. Ont Health Technol Assess Ser. 2016. PMID: 27099644 Free PMC article. Review.
Cited by
-
Outcome of patients treated for relapsed or refractory acute lymphoblastic leukemia: a Therapeutic Advances in Childhood Leukemia Consortium study.J Clin Oncol. 2010 Feb 1;28(4):648-54. doi: 10.1200/JCO.2009.22.2950. Epub 2009 Oct 19. J Clin Oncol. 2010. PMID: 19841326 Free PMC article.
-
Replacing cyclophosphamide/cytarabine/mercaptopurine with cyclophosphamide/etoposide during consolidation/delayed intensification does not improve outcome for pediatric B-cell acute lymphoblastic leukemia: a report from the COG.Haematologica. 2019 May;104(5):986-992. doi: 10.3324/haematol.2018.204545. Epub 2018 Dec 13. Haematologica. 2019. PMID: 30545921 Free PMC article. Clinical Trial.
-
Hematopoietic stem cell transplantation for leukemia.Pediatr Clin North Am. 2010 Feb;57(1):1-25. doi: 10.1016/j.pcl.2009.11.005. Pediatr Clin North Am. 2010. PMID: 20307709 Free PMC article. Review.
-
Toxicity assessment of molecularly targeted drugs incorporated into multiagent chemotherapy regimens for pediatric acute lymphocytic leukemia (ALL): review from an international consensus conference.Pediatr Blood Cancer. 2010 Jul 1;54(7):872-8. doi: 10.1002/pbc.22414. Pediatr Blood Cancer. 2010. PMID: 20127846 Free PMC article.
-
New frontiers in pediatric Allo-SCT: novel approaches for children and adolescents with ALL.Bone Marrow Transplant. 2014 Oct;49(10):1259-65. doi: 10.1038/bmt.2014.114. Epub 2014 Jun 16. Bone Marrow Transplant. 2014. PMID: 24933210 Review.
References
-
- Saarinen-Pihkala UM, Heilmann C, Winiarski J, et al: Pathways through relapses and deaths of children with acute lymphoblastic leukemia: Role of allogeneic stem-cell transplantation in Nordic data. J Clin Oncol 24:5750-5762, 2006 - PubMed
-
- Gaynon PS, Harris RE, Altman AJ, et al: Bone marrow transplantation versus prolonged intensive chemotherapy for children with acute lymphoblastic leukemia and an initial bone marrow relapse within 12 months of the completion of primary therapy: Children's Oncology Group study CCG-1941. J Clin Oncol 24:3150-3156, 2006 - PubMed
-
- Abshire TC, Pollock BH, Billett AL, et al: Weekly polyethylene glycol conjugated L-asparaginase compared with biweekly dosing produces superior induction remission rates in childhood relapsed acute lymphoblastic leukemia: A Pediatric Oncology Group study. Blood 96:1709-1715, 2000 - PubMed
-
- Buchanan GR, Rivera GK, Boyett JM, et al: Reinduction therapy in 297 children with acute lymphoblastic leukemia in first bone marrow relapse: A Pediatric Oncology Group study. Blood 72:1286-1292, 1988 - PubMed
-
- Einsiedel HG, von Stackelberg A, Hartmann R, et al: Long-term outcome in children with relapsed ALL by risk-stratified salvage therapy: Results of trial acute lymphoblastic leukemia-relapse study of the Berlin-Frankfurt-Munster Group 87. J Clin Oncol 23:7942-7950, 2005 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

