Histone deacetylase inhibitors modify pancreatic cell fate determination and amplify endocrine progenitors
- PMID: 18710955
- PMCID: PMC2577441
- DOI: 10.1128/MCB.00413-08
Histone deacetylase inhibitors modify pancreatic cell fate determination and amplify endocrine progenitors
Abstract
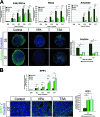
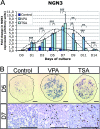
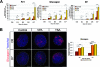
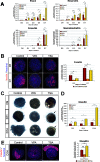
During pancreas development, transcription factors play critical roles in exocrine and endocrine differentiation. Transcriptional regulation in eukaryotes occurs within chromatin and is influenced by posttranslational histone modifications (e.g., acetylation) involving histone deacetylases (HDACs). Here, we show that HDAC expression and activity are developmentally regulated in the embryonic rat pancreas. We discovered that pancreatic treatment with different HDAC inhibitors (HDACi) modified the timing and determination of pancreatic cell fate. HDACi modified the exocrine lineage via abolition and enhancement of acinar and ductal differentiation, respectively. Importantly, HDACi treatment promoted the NGN3 proendocrine lineage, leading to an increased pool of endocrine progenitors and modified endocrine subtype lineage choices. Interestingly, treatments with trichostatin A and sodium butyrate, two inhibitors of both class I and class II HDACs, enhanced the pool of beta cells. These results highlight the roles of HDACs at key points in exocrine and endocrine differentiation. They show the powerful use of HDACi to switch pancreatic cell determination and amplify specific cellular subtypes, with potential applications in cell replacement therapies in diabetes.
Figures



Similar articles
-
Presenilins, Notch dose control the fate of pancreatic endocrine progenitors during a narrow developmental window.Genes Dev. 2009 Sep 1;23(17):2088-101. doi: 10.1101/gad.1800209. Genes Dev. 2009. PMID: 19723764 Free PMC article.
-
Direct lineage tracing reveals the ontogeny of pancreatic cell fates during mouse embryogenesis.Mech Dev. 2003 Jan;120(1):35-43. doi: 10.1016/s0925-4773(02)00330-1. Mech Dev. 2003. PMID: 12490294 Review.
-
Glucose is necessary for embryonic pancreatic endocrine cell differentiation.J Biol Chem. 2007 May 18;282(20):15228-37. doi: 10.1074/jbc.M610986200. Epub 2007 Mar 21. J Biol Chem. 2007. PMID: 17376780
-
Differential effects of class I isoform histone deacetylase depletion and enzymatic inhibition by belinostat or valproic acid in HeLa cells.Mol Cancer. 2008 Sep 12;7:70. doi: 10.1186/1476-4598-7-70. Mol Cancer. 2008. PMID: 18789133 Free PMC article.
-
Lineage commitment and cellular differentiation in exocrine pancreas.Pancreatology. 2001;1(6):587-96. doi: 10.1159/000055868. Pancreatology. 2001. PMID: 12120241 Review.
Cited by
-
Histone Acetylation and Its Modifiers in the Pathogenesis of Diabetic Nephropathy.J Diabetes Res. 2016;2016:4065382. doi: 10.1155/2016/4065382. Epub 2016 Jun 9. J Diabetes Res. 2016. PMID: 27379253 Free PMC article. Review.
-
Deciphering early human pancreas development at the single-cell level.Nat Commun. 2023 Sep 2;14(1):5354. doi: 10.1038/s41467-023-40893-8. Nat Commun. 2023. PMID: 37660175 Free PMC article.
-
Nutrition, epigenetics, and metabolic syndrome.Antioxid Redox Signal. 2012 Jul 15;17(2):282-301. doi: 10.1089/ars.2011.4381. Epub 2012 Jan 13. Antioxid Redox Signal. 2012. PMID: 22044276 Free PMC article. Review.
-
Small-molecule inhibitors of the cystic fibrosis transmembrane conductance regulator increase pancreatic endocrine cell development in rat and mouse.Diabetologia. 2013 Feb;56(2):330-9. doi: 10.1007/s00125-012-2778-8. Epub 2012 Nov 24. Diabetologia. 2013. PMID: 23178930 Free PMC article.
-
Proteomic basis for pancreatic acinar cell carcinoma and pancreatoblastoma as similar yet distinct entities.NPJ Precis Oncol. 2024 Oct 3;8(1):221. doi: 10.1038/s41698-024-00708-5. NPJ Precis Oncol. 2024. PMID: 39363045 Free PMC article.
References
-
- Apelqvist, A., H. Li, L. Sommer, P. Beatus, D. J. Anderson, T. Honjo, M. Hrabe de Angelis, U. Lendahl, and H. Edlund. 1999. Notch signalling controls pancreatic cell differentiation. Nature 400877-881. - PubMed
-
- Attali, M., V. Stetsyuk, A. Basmaciogullari, V. Aiello, M. A. Zanta-Boussif, B. Duvillie, and R. Scharfmann. 2007. Control of beta-cell differentiation by the pancreatic mesenchyme. Diabetes 561248-1258. - PubMed
-
- Backs, J., and E. N. Olson. 2006. Control of cardiac growth by histone acetylation/deacetylation. Circ. Res. 9815-24. - PubMed
-
- Bhushan, A., N. Itoh, S. Kato, J. P. Thiery, P. Czernichow, S. Bellusci, and R. Scharfmann. 2001. Fgf10 is essential for maintaining the proliferative capacity of epithelial progenitor cells during early pancreatic organogenesis. Development 1285109-5117. - PubMed
-
- Cano, D. A., S. Sekine, and M. Hebrok. 2006. Primary cilia deletion in pancreatic epithelial cells results in cyst formation and pancreatitis. Gastroenterology 1311856-1869. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
