A novel purification method for CNS projection neurons leads to the identification of brain vascular cells as a source of trophic support for corticospinal motor neurons
- PMID: 18701692
- PMCID: PMC2567869
- DOI: 10.1523/JNEUROSCI.2010-08.2008
A novel purification method for CNS projection neurons leads to the identification of brain vascular cells as a source of trophic support for corticospinal motor neurons
Abstract
One of the difficulties in studying cellular interactions in the CNS is the lack of effective methods to purify specific neuronal populations of interest. We report the development of a novel purification scheme, cholera toxin beta (CTB) immunopanning, in which a particular CNS neuron population is selectively labeled via retrograde axonal transport of the cell-surface epitope CTB, and then purified via immobilization with anti-CTB antibody. We have demonstrated the usefulness and versatility of this method by purifying both retinal ganglion cells and corticospinal motor neurons (CSMNs). Genomic expression analyses of purified CSMNs revealed that they express significant levels of many receptors for growth factors produced by brain endothelial cells; three of these factors, CXCL12, pleiotrophin, and IGF2 significantly enhanced purified CSMN survival, similar to previously characterized CSMN trophic factors BDNF and IGF1. In addition, endothelial cell conditioned medium significantly promoted CSMN neurite outgrowth. These findings demonstrate a useful method for the purification of several different types of CNS projection neurons, which in principle should work in many mammalian species, and provide evidence that endothelial-derived factors may represent an overlooked source of trophic support for neurons in the brain.
Figures
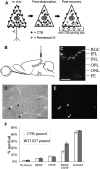

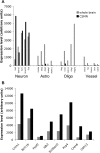
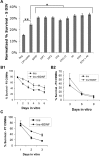
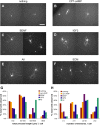
Similar articles
-
Immunopanning of retrograde-labeled corticospinal motor neurons from early postnatal rodents.Cold Spring Harb Protoc. 2014 Apr 1;2014(4):375-88. doi: 10.1101/pdb.prot074930. Cold Spring Harb Protoc. 2014. PMID: 24692491
-
CNTF and GDNF, but not NT-4, support corticospinal motor neuron growth via direct mechanisms.Neuroreport. 1998 Nov 16;9(16):3749-54. doi: 10.1097/00001756-199811160-00033. Neuroreport. 1998. PMID: 9858391
-
Retrograde labeling of corticospinal motor neurons from early postnatal rodents.Cold Spring Harb Protoc. 2014 Apr 1;2014(4):372-9. doi: 10.1101/pdb.prot074922. Cold Spring Harb Protoc. 2014. PMID: 24692490
-
Purification and culture of corticospinal motor neurons.Cold Spring Harb Protoc. 2014 Apr 1;2014(4):336-8. doi: 10.1101/pdb.top070938. Cold Spring Harb Protoc. 2014. PMID: 24692486 Review.
-
Trophic dependencies of rodent corticospinal neurons.Rev Neurosci. 2001;12(1):79-94. doi: 10.1515/revneuro.2001.12.1.79. Rev Neurosci. 2001. PMID: 11236067 Review.
Cited by
-
Vascular endothelial growth factor regulates the migration of oligodendrocyte precursor cells.J Neurosci. 2011 Jul 20;31(29):10666-70. doi: 10.1523/JNEUROSCI.1944-11.2011. J Neurosci. 2011. PMID: 21775609 Free PMC article.
-
Chemokines, macrophage inflammatory protein-2 and stromal cell-derived factor-1α, suppress amyloid β-induced neurotoxicity.Toxicol Appl Pharmacol. 2011 Nov 1;256(3):300-13. doi: 10.1016/j.taap.2011.06.006. Epub 2011 Jun 17. Toxicol Appl Pharmacol. 2011. PMID: 21704645 Free PMC article.
-
Quantitative biology of single neurons.J R Soc Interface. 2012 Dec 7;9(77):3165-83. doi: 10.1098/rsif.2012.0417. Epub 2012 Aug 22. J R Soc Interface. 2012. PMID: 22915636 Free PMC article. Review.
-
New insights in ferroptosis: Potential therapeutic targets for the treatment of ischemic stroke.Front Pharmacol. 2022 Nov 8;13:1020918. doi: 10.3389/fphar.2022.1020918. eCollection 2022. Front Pharmacol. 2022. PMID: 36425577 Free PMC article. Review.
-
Edaravone, a free radical scavenger, protects components of the neurovascular unit against oxidative stress in vitro.Brain Res. 2010 Jan 11;1307:22-7. doi: 10.1016/j.brainres.2009.10.026. Epub 2009 Oct 17. Brain Res. 2010. PMID: 19840779 Free PMC article.
References
-
- Aghajanian GK, Rasmussen K. Intracellular studies in the facial nucleus illustrating a simple new method for obtaining viable motoneurons in adult rat brain slices. Synapse. 1989;3:331–338. - PubMed
-
- Arlotta P, Molyneaux BJ, Chen J, Inoue J, Kominami R, Macklis JD. Neuronal subtype-specific genes that control corticospinal motor neuron development in vivo. Neuron. 2005;45:207–221. - PubMed
-
- Bareyre FM, Kerschensteiner M, Misgeld T, Sanes JR. Transgenic labeling of the corticospinal tract for monitoring axonal responses to spinal cord injury. Nat Med. 2005;11:1355–1360. - PubMed
-
- Barres BA, Silverstein BE, Corey DP, Chun LL. Immunological, morphological, and electrophysiological variation among retinal ganglion cells purified by panning. Neuron. 1988;1:791–803. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
