Gr1(+) inflammatory monocytes are required for mucosal resistance to the pathogen Toxoplasma gondii
- PMID: 18691912
- PMCID: PMC2605393
- DOI: 10.1016/j.immuni.2008.05.019
Gr1(+) inflammatory monocytes are required for mucosal resistance to the pathogen Toxoplasma gondii
Erratum in
- Immunity. 2008 Oct;29(4):660
Abstract
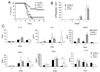
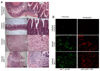
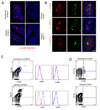
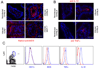
The enteric pathogen Toxoplasma gondii is controlled by a vigorous innate T helper 1 (Th1) cell response in the murine model. We demonstrated that after oral infection, the parasite rapidly recruited inflammatory monocytes [Gr1(+) (Ly6C(+), Ly6G(-)) F4/80(+)CD11b(+)CD11c(-)], which established a vital defensive perimeter within the villi of the ileum in the small intestine. Mice deficient of the chemokine receptor CCR2 or the ligand CCL2 failed to recruit Gr1(+) inflammatory monocytes, whereas dendritic cells and resident tissue macrophages remained unaltered. The selective lack of Gr1(+) inflammatory monocytes resulted in an inability of mice to control replication of the parasite, high influx of neutrophils, extensive intestinal necrosis, and rapid death. Adoptive transfer of sorted Gr1(+) inflammatory monocytes demonstrated their ability to home to the ileum in infected animals and protect Ccr2(-/-) mice, which were otherwise highly susceptible to oral toxoplasmosis. Collectively, these findings illustrate the critical importance of inflammatory monocytes as a first line of defense in controlling intestinal pathogens.
Figures



Similar articles
-
Toxoplasma gondii profilin promotes recruitment of Ly6Chi CCR2+ inflammatory monocytes that can confer resistance to bacterial infection.PLoS Pathog. 2014 Jun 12;10(6):e1004203. doi: 10.1371/journal.ppat.1004203. eCollection 2014 Jun. PLoS Pathog. 2014. PMID: 24945711 Free PMC article.
-
Recruitment of Gr-1+ monocytes is essential for control of acute toxoplasmosis.J Exp Med. 2005 Jun 6;201(11):1761-9. doi: 10.1084/jem.20050054. Epub 2005 May 31. J Exp Med. 2005. PMID: 15928200 Free PMC article.
-
Inflammatory monocytes but not neutrophils are necessary to control infection with Toxoplasma gondii in mice.Infect Immun. 2010 Apr;78(4):1564-70. doi: 10.1128/IAI.00472-09. Epub 2010 Feb 9. Infect Immun. 2010. PMID: 20145099 Free PMC article.
-
Insights into inflammatory bowel disease using Toxoplasma gondii as an infectious trigger.Immunol Cell Biol. 2012 Aug;90(7):668-75. doi: 10.1038/icb.2011.93. Epub 2011 Nov 8. Immunol Cell Biol. 2012. PMID: 22064707 Free PMC article. Review.
-
Monocytes mediate mucosal immunity to Toxoplasma gondii.Curr Opin Immunol. 2010 Aug;22(4):461-6. doi: 10.1016/j.coi.2010.04.008. Epub 2010 May 27. Curr Opin Immunol. 2010. PMID: 20537517 Free PMC article. Review.
Cited by
-
Use and abuse of dendritic cells by Toxoplasma gondii.Virulence. 2012 Nov 15;3(7):678-89. doi: 10.4161/viru.22833. Epub 2012 Nov 15. Virulence. 2012. PMID: 23221473 Free PMC article. Review.
-
Extramedullary myelopoiesis in malaria depends on mobilization of myeloid-restricted progenitors by IFN-γ induced chemokines.PLoS Pathog. 2013;9(6):e1003406. doi: 10.1371/journal.ppat.1003406. Epub 2013 Jun 6. PLoS Pathog. 2013. PMID: 23762028 Free PMC article.
-
The secreted kinase ROP17 promotes Toxoplasma gondii dissemination by hijacking monocyte tissue migration.Nat Microbiol. 2019 Nov;4(11):1951-1963. doi: 10.1038/s41564-019-0504-8. Epub 2019 Jul 22. Nat Microbiol. 2019. PMID: 31332383 Free PMC article.
-
Virulence of Toxoplasma gondii is associated with distinct dendritic cell responses and reduced numbers of activated CD8+ T cells.J Immunol. 2010 Aug 1;185(3):1502-12. doi: 10.4049/jimmunol.0903450. Epub 2010 Jun 30. J Immunol. 2010. PMID: 20592284 Free PMC article.
-
Inflammatory monocytes promote granuloma control of Yersinia infection.Nat Microbiol. 2023 Apr;8(4):666-678. doi: 10.1038/s41564-023-01338-6. Epub 2023 Mar 6. Nat Microbiol. 2023. PMID: 36879169 Free PMC article.
References
-
- Aliberti J, Sousa CR, Schito M, Hieny S, Wells T, Huffnagle GB, Sher A. CCR5 provides a signal for microbial induced production of IL-12 by CD8-α+ dendritic cells. Nat. Immunol. 2000;1:83–87. - PubMed
-
- Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, Sarnacki S, Cumano A, Lauvau G, Geissmann F. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317:666–670. - PubMed
-
- Barragan A, Sibley LD. Migration of Toxoplasma gondii across biological barriers. Trends Microbiol. 2003;11:426–430. - PubMed
-
- Bliss SK, Marshall AJ, Zhang Y, Denkers EY. Human polymorphonuclear leukocytes produce IL-12, TNF-α, and the chemokines macrophage-inflammatory protein-1α and -1β in response to Toxoplasma gondii antigens. J. Immunol. 1999;162:7369–7375. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

