PKC1 is essential for protection against both oxidative and nitrosative stresses, cell integrity, and normal manifestation of virulence factors in the pathogenic fungus Cryptococcus neoformans
- PMID: 18689526
- PMCID: PMC2568057
- DOI: 10.1128/EC.00146-08
PKC1 is essential for protection against both oxidative and nitrosative stresses, cell integrity, and normal manifestation of virulence factors in the pathogenic fungus Cryptococcus neoformans
Abstract
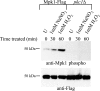
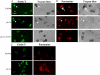
Cell wall integrity is crucial for fungal growth, survival, and pathogenesis. Responses to environmental stresses are mediated by the highly conserved Pkc1 protein and its downstream components. In this study, we demonstrate that both oxidative and nitrosative stresses activate the PKC1 cell integrity pathway in wild-type cells, as measured by phosphorylation of Mpk1, the terminal protein in the PKC1 phosphorylation cascade. Furthermore, deletion of PKC1 shows that this gene is essential for defense against both oxidative and nitrosative stresses; however, other genes involved directly in the PKC1 pathway are dispensable for protection against these stresses. This suggests that Pkc1 may have multiple and alternative functions other than activating the mitogen-activated protein kinase cascade from a "top-down" approach. Deletion of PKC1 also causes osmotic instability, temperature sensitivity, severe sensitivity to cell wall-inhibiting agents, and alterations in capsule and melanin. Furthermore, the vital cell wall components chitin and its deacetylated form chitosan appear to be mislocalized in a pkc1Delta strain, although this mutant contains wild-type levels of both of these polymers. These data indicate that loss of Pkc1 has pleiotropic effects because it is central to many functions either dependent on or independent of PKC1 pathway activation. Notably, this is the first time that Pkc1 has been implicated in protection against nitrosative stress in any organism.
Figures





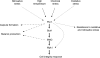
Similar articles
-
A novel specificity protein 1 (SP1)-like gene regulating protein kinase C-1 (Pkc1)-dependent cell wall integrity and virulence factors in Cryptococcus neoformans.J Biol Chem. 2011 Jun 10;286(23):20977-90. doi: 10.1074/jbc.M111.230268. Epub 2011 Apr 12. J Biol Chem. 2011. PMID: 21487010 Free PMC article.
-
Role of Cryptococcus neoformans Rho1 GTPases in the PKC1 signaling pathway in response to thermal stress.Eukaryot Cell. 2013 Jan;12(1):118-31. doi: 10.1128/EC.05305-11. Epub 2012 Nov 16. Eukaryot Cell. 2013. PMID: 23159519 Free PMC article.
-
The casein kinase I protein Cck1 regulates multiple signaling pathways and is essential for cell integrity and fungal virulence in Cryptococcus neoformans.Eukaryot Cell. 2011 Nov;10(11):1455-64. doi: 10.1128/EC.05207-11. Epub 2011 Sep 16. Eukaryot Cell. 2011. PMID: 21926330 Free PMC article.
-
[Pathogenicity of the opportunistic pathogenic fungus Cryptococcus neoformans--a review].Wei Sheng Wu Xue Bao. 2009 Apr;49(4):423-8. Wei Sheng Wu Xue Bao. 2009. PMID: 19621627 Review. Chinese.
-
Emerging themes in cryptococcal capsule synthesis.Curr Opin Struct Biol. 2011 Oct;21(5):597-602. doi: 10.1016/j.sbi.2011.08.006. Epub 2011 Sep 1. Curr Opin Struct Biol. 2011. PMID: 21889889 Free PMC article. Review.
Cited by
-
What Are the Best Parents for Hybrid Progeny? An Investigation into the Human Pathogenic Fungus Cryptococcus.J Fungi (Basel). 2021 Apr 15;7(4):299. doi: 10.3390/jof7040299. J Fungi (Basel). 2021. PMID: 33920829 Free PMC article.
-
Phospholipase C of Cryptococcus neoformans regulates homeostasis and virulence by providing inositol trisphosphate as a substrate for Arg1 kinase.Infect Immun. 2013 Apr;81(4):1245-55. doi: 10.1128/IAI.01421-12. Epub 2013 Feb 4. Infect Immun. 2013. PMID: 23381992 Free PMC article.
-
The TOR Pathway Plays Pleiotropic Roles in Growth and Stress Responses of the Fungal Pathogen Cryptococcus neoformans.Genetics. 2019 Aug;212(4):1241-1258. doi: 10.1534/genetics.119.302191. Epub 2019 Jun 7. Genetics. 2019. PMID: 31175227 Free PMC article.
-
How sweet it is! Cell wall biogenesis and polysaccharide capsule formation in Cryptococcus neoformans.Annu Rev Microbiol. 2009;63:223-47. doi: 10.1146/annurev.micro.62.081307.162753. Annu Rev Microbiol. 2009. PMID: 19575556 Free PMC article. Review.
-
Concepts and principles of photodynamic therapy as an alternative antifungal discovery platform.Front Microbiol. 2012 Apr 10;3:120. doi: 10.3389/fmicb.2012.00120. eCollection 2012. Front Microbiol. 2012. PMID: 22514547 Free PMC article.
References
-
- Alspaugh, J. A., R. Pukkila-Worley, T. Harashima, L. M. Cavallo, D. Funnell, G. M. Cox, J. R. Perfect, J. W. Kronstad, and J. Heitman. 2002. Adenylyl cyclase functions downstream of the Gα protein Gpa1 and controls mating and pathogenicity of Cryptococcus neoformans. Eukaryot. Cell 175-84. - PMC - PubMed
-
- Bartnicki-Garcia, S. 2006. Chitosomes: past, present and future. FEMS Yeast Res. 6957-965. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

