The intermediate-conductance calcium-activated potassium channel KCa3.1 contributes to atherogenesis in mice and humans
- PMID: 18688283
- PMCID: PMC2496961
- DOI: 10.1172/JCI30836
The intermediate-conductance calcium-activated potassium channel KCa3.1 contributes to atherogenesis in mice and humans
Abstract
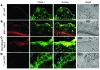
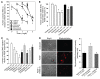
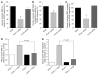
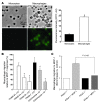
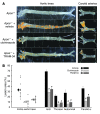
Atherosclerosis remains a major cause of death in the developed world despite the success of therapies that lower cholesterol and BP. The intermediate-conductance calcium-activated potassium channel KCa3.1 is expressed in multiple cell types implicated in atherogenesis, and pharmacological blockade of this channel inhibits VSMC and lymphocyte activation in rats and mice. We found that coronary vessels from patients with coronary artery disease expressed elevated levels of KCa3.1. In Apoe(-/-) mice, a genetic model of atherosclerosis, KCa3.1 expression was elevated in the VSMCs, macrophages, and T lymphocytes that infiltrated atherosclerotic lesions. Selective pharmacological blockade and gene silencing of KCa3.1 suppressed proliferation, migration, and oxidative stress of human VSMCs. Furthermore, VSMC proliferation and macrophage activation were reduced in KCa3.1(-/-) mice. In vivo therapy with 2 KCa3.1 blockers, TRAM-34 and clotrimazole, significantly reduced the development of atherosclerosis in aortas of Apoe(-/-) mice by suppressing VSMC proliferation and migration into plaques, decreasing infiltration of plaques by macrophages and T lymphocytes, and reducing oxidative stress. Therapeutic concentrations of TRAM-34 in mice caused no discernible toxicity after repeated dosing and did not compromise the immune response to influenza virus. These data suggest that KCa3.1 blockers represent a promising therapeutic strategy for atherosclerosis.
Figures

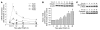
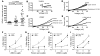

Similar articles
-
The intermediate conductance calcium-activated potassium channel KCa3.1 regulates vascular smooth muscle cell proliferation via controlling calcium-dependent signaling.J Biol Chem. 2013 May 31;288(22):15843-53. doi: 10.1074/jbc.M112.427187. Epub 2013 Apr 22. J Biol Chem. 2013. PMID: 23609438 Free PMC article.
-
The potassium channel KCa3.1 as new therapeutic target for the prevention of obliterative airway disease.Transplantation. 2013 Jan 27;95(2):285-92. doi: 10.1097/TP.0b013e318275a2f4. Transplantation. 2013. PMID: 23325003 Free PMC article.
-
Inhibition of vascular calcification by block of intermediate conductance calcium-activated potassium channels with TRAM-34.Pharmacol Res. 2014 Jul;85:6-14. doi: 10.1016/j.phrs.2014.04.013. Epub 2014 May 6. Pharmacol Res. 2014. PMID: 24813858
-
Critical regulation of atherosclerosis by the KCa3.1 channel and the retargeting of this therapeutic target in in-stent neoatherosclerosis.J Mol Med (Berl). 2019 Sep;97(9):1219-1229. doi: 10.1007/s00109-019-01814-9. Epub 2019 Jun 28. J Mol Med (Berl). 2019. PMID: 31254004 Review.
-
The role of T cell potassium channels, KV1.3 and KCa3.1, in the inflammatory cascade in ulcerative colitis.Dan Med J. 2014 Nov;61(11):B4946. Dan Med J. 2014. PMID: 25370966 Review.
Cited by
-
The Lymphocyte Potassium Channels Kv1.3 and KCa3.1 as Targets for Immunosuppression.Drug Dev Res. 2011 Nov;72(7):573-584. doi: 10.1002/ddr.20467. Drug Dev Res. 2011. PMID: 22241939 Free PMC article.
-
Blockade of KCa3.1 ameliorates renal fibrosis through the TGF-β1/Smad pathway in diabetic mice.Diabetes. 2013 Aug;62(8):2923-34. doi: 10.2337/db13-0135. Epub 2013 May 8. Diabetes. 2013. PMID: 23656889 Free PMC article.
-
The KCa3.1 blocker TRAM-34 reduces infarction and neurological deficit in a rat model of ischemia/reperfusion stroke.J Cereb Blood Flow Metab. 2011 Dec;31(12):2363-74. doi: 10.1038/jcbfm.2011.101. Epub 2011 Jul 13. J Cereb Blood Flow Metab. 2011. PMID: 21750563 Free PMC article.
-
Calcium-activated potassium channel KCa3.1 in lung dendritic cell migration.Am J Respir Cell Mol Biol. 2011 Nov;45(5):962-8. doi: 10.1165/rcmb.2010-0514OC. Epub 2011 Apr 14. Am J Respir Cell Mol Biol. 2011. PMID: 21493782 Free PMC article.
-
Upregulation of intermediate-conductance Ca2+-activated K+ channels (KCNN4) in porcine coronary smooth muscle requires NADPH oxidase 5 (NOX5).PLoS One. 2014 Aug 21;9(8):e105337. doi: 10.1371/journal.pone.0105337. eCollection 2014. PLoS One. 2014. PMID: 25144362 Free PMC article.
References
-
- Neylon C.B., Lang R.J., Fu Y., Bobik A., Reinhart P.H. Molecular cloning and characterization of the intermediate-conductance Ca2+-activated K+ channel in vascular smooth muscle: relationship between KCa channel diversity and smooth muscle cell function. . Circ. Res. 1999;85:e33–e43. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DE09692/DE/NIDCR NIH HHS/United States
- R37 DE008921/DE/NIDCR NIH HHS/United States
- R01 HL080173-02/HL/NHLBI NIH HHS/United States
- R01 DE009692/DE/NIDCR NIH HHS/United States
- P01 HL068769/HL/NHLBI NIH HHS/United States
- R01 HL080173-01A2/HL/NHLBI NIH HHS/United States
- R01 NS48252/NS/NINDS NIH HHS/United States
- P50 HL65203/HL/NHLBI NIH HHS/United States
- P50 HL065203/HL/NHLBI NIH HHS/United States
- R01 NS048252/NS/NINDS NIH HHS/United States
- P01 HL68769-02/HL/NHLBI NIH HHS/United States
- P01 HL059996/HL/NHLBI NIH HHS/United States
- R01 GM076063/GM/NIGMS NIH HHS/United States
- P01 HL59996/HL/NHLBI NIH HHS/United States
- R01 HL094971/HL/NHLBI NIH HHS/United States
- R37 DE08921/DE/NIDCR NIH HHS/United States
- R01 HL080173/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

