Cadherins and catenins at synapses: roles in synaptogenesis and synaptic plasticity
- PMID: 18684518
- PMCID: PMC2623250
- DOI: 10.1016/j.tins.2008.07.001
Cadherins and catenins at synapses: roles in synaptogenesis and synaptic plasticity
Abstract
Synapse formation involves reciprocal interactions between cells resulting in formation of a structure optimized for efficient information transfer. Recent work has implicated constituents of the cadherin-catenin cell-adhesion complex in both synapse formation and plasticity. In this review, we describe recent interesting discoveries on mechanisms of cadherin complex function, in addition to regulating adhesion, that are relevant for understanding the role of this complex in synaptogenesis and plasticity. We describe how this complex acts via (i) recruitment/stabilization of intracellular partners; (ii) regulation of intracellular signaling pathways; (iii) regulation of cadherin surface levels, stability and turnover; (iv) stabilization of receptors; and (v) regulation of gene expression. These exciting discoveries provide insights into novel functional roles of the complex beyond regulating cell adhesion.
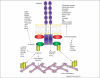
Figures

Similar articles
-
Cadherins and catenins in dendrite and synapse morphogenesis.Cell Adh Migr. 2015;9(3):202-13. doi: 10.4161/19336918.2014.994919. Cell Adh Migr. 2015. PMID: 25914083 Free PMC article. Review.
-
Regulation of dendrite and spine morphogenesis and plasticity by catenins.Mol Neurobiol. 2009 Aug;40(1):46-54. doi: 10.1007/s12035-009-8068-x. Epub 2009 Apr 29. Mol Neurobiol. 2009. PMID: 19401831 Review.
-
Cadherins in neuronal morphogenesis and function.Dev Growth Differ. 2008 Jun;50 Suppl 1:S119-30. doi: 10.1111/j.1440-169X.2008.01002.x. Epub 2008 Apr 22. Dev Growth Differ. 2008. PMID: 18430170 Review.
-
Palmitoylation of δ-catenin by DHHC5 mediates activity-induced synapse plasticity.Nat Neurosci. 2014 Apr;17(4):522-32. doi: 10.1038/nn.3657. Epub 2014 Feb 23. Nat Neurosci. 2014. PMID: 24562000 Free PMC article.
-
The role of cadherins and catenins in gliomagenesis.Neurosurg Focus. 2006 Oct 15;21(4):E13. doi: 10.3171/foc.2006.21.4.14. Neurosurg Focus. 2006. PMID: 17112191 Review.
Cited by
-
Cadherin-based transsynaptic networks in establishing and modifying neural connectivity.Curr Top Dev Biol. 2015;112:415-65. doi: 10.1016/bs.ctdb.2014.11.025. Epub 2015 Feb 11. Curr Top Dev Biol. 2015. PMID: 25733148 Free PMC article. Review.
-
Expression of delta-catenin is associated with progression of human astrocytoma.BMC Cancer. 2011 Dec 12;11:514. doi: 10.1186/1471-2407-11-514. BMC Cancer. 2011. PMID: 22151302 Free PMC article.
-
Direct and Indirect Regulation of Spinal Cord Ia Afferent Terminal Formation by the γ-Protocadherins.Front Mol Neurosci. 2011 Dec 23;4:54. doi: 10.3389/fnmol.2011.00054. eCollection 2011. Front Mol Neurosci. 2011. PMID: 22275881 Free PMC article.
-
Striatal transcriptome of a mouse model of ADHD reveals a pattern of synaptic remodeling.PLoS One. 2018 Aug 15;13(8):e0201553. doi: 10.1371/journal.pone.0201553. eCollection 2018. PLoS One. 2018. PMID: 30110355 Free PMC article.
-
miR-29a Negatively Affects Glucose-Stimulated Insulin Secretion and MIN6 Cell Proliferation via Cdc42/β-Catenin Signaling.Int J Endocrinol. 2019 Aug 28;2019:5219782. doi: 10.1155/2019/5219782. eCollection 2019. Int J Endocrinol. 2019. PMID: 31662747 Free PMC article.
References
-
- Yuste R, Bonhoeffer T. Morphological changes in dendritic spines associated with long-term synaptic plasticity. Annu. Rev. Neurosci. 2001;24:1071–1089. - PubMed
-
- Alvarez VA, Sabatini BL. Anatomical and physiological plasticity of dendritic spines. Annu. Rev. Neurosci. 2007;30:79–97. - PubMed
-
- Bourne J, Harris KM. Do thin spines learn to be mushroom spines that remember? Curr. Opin. Neurobiol. 2007;17:381–386. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

