CSIG inhibits PTEN translation in replicative senescence
- PMID: 18678645
- PMCID: PMC2577433
- DOI: 10.1128/MCB.00142-08
CSIG inhibits PTEN translation in replicative senescence
Abstract
Using a suppressive subtractive hybridization system, we identified CSIG (cellular senescence-inhibited gene protein; RSL1D1) that was abundant in young human diploid fibroblast cells but declined upon replicative senescence. Overexpression or knockdown of CSIG did not influence p21(Cip1) and p16(INK4a) expressions. Instead, CSIG negatively regulated PTEN and p27(Kip1) expressions, in turn promoting cell proliferation. In PTEN-silenced HEK 293 cells and PTEN-deficient human glioblastoma U87MG cells, the effect of CSIG on p27(Kip1) expression and cell division was abolished, suggesting that PTEN was required for the role of CSIG on p27(Kip1) regulation and cell cycle progression. Investigation into the underlying mechanism revealed that the regulation of PTEN by CSIG was achieved through a translational suppression mechanism. Further study showed that CSIG interacted with PTEN mRNA in the 5' untranslated region (UTR) and that knockdown of CSIG led to increased luciferase activity of a PTEN 5' UTR-luciferase reporter. Moreover, overexpression of CSIG significantly delayed the progression of replicative senescence, while knockdown of CSIG expression accelerated replicative senescence. Knockdown of PTEN diminished the effect of CSIG on cellular senescence. Our findings indicate that CSIG acts as a novel regulatory component of replicative senescence, which requires PTEN as a mediator and involves in a translational regulatory mechanism.
Figures
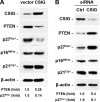

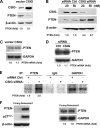
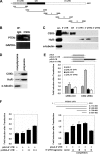


Similar articles
-
Ribosomal L1 domain and lysine-rich region are essential for CSIG/ RSL1D1 to regulate proliferation and senescence.Biochem Biophys Res Commun. 2016 Jan 15;469(3):593-8. doi: 10.1016/j.bbrc.2015.12.004. Epub 2015 Dec 12. Biochem Biophys Res Commun. 2016. PMID: 26686419
-
RSL1D1 modulates cell senescence and proliferation via regulation of PPARγ mRNA stability.Life Sci. 2022 Oct 15;307:120848. doi: 10.1016/j.lfs.2022.120848. Epub 2022 Aug 5. Life Sci. 2022. PMID: 35940221
-
NSun2 delays replicative senescence by repressing p27 (KIP1) translation and elevating CDK1 translation.Aging (Albany NY). 2015 Dec;7(12):1143-58. doi: 10.18632/aging.100860. Aging (Albany NY). 2015. PMID: 26687548 Free PMC article.
-
[Advance research on cellular senescence-inhibited gene (CSIG)].Sheng Li Ke Xue Jin Zhan. 2012 Aug;43(4):291-3. Sheng Li Ke Xue Jin Zhan. 2012. PMID: 23189626 Review. Chinese. No abstract available.
-
Tumor suppressors and oncogenes in cellular senescence.Exp Gerontol. 2000 May;35(3):317-29. doi: 10.1016/s0531-5565(00)00083-8. Exp Gerontol. 2000. PMID: 10832053 Review.
Cited by
-
Toward understanding the genetic basis of adaptation to high-elevation life in poikilothermic species: a comparative transcriptomic analysis of two ranid frogs, Rana chensinensis and R. kukunoris.BMC Genomics. 2012 Nov 1;13:588. doi: 10.1186/1471-2164-13-588. BMC Genomics. 2012. PMID: 23116153 Free PMC article.
-
CSIG promotes hepatocellular carcinoma proliferation by activating c-MYC expression.Oncotarget. 2015 Mar 10;6(7):4733-44. doi: 10.18632/oncotarget.2900. Oncotarget. 2015. PMID: 25749381 Free PMC article.
-
The Complex Landscape of PTEN mRNA Regulation.Cold Spring Harb Perspect Med. 2020 Jun 1;10(6):a036236. doi: 10.1101/cshperspect.a036236. Cold Spring Harb Perspect Med. 2020. PMID: 31871240 Free PMC article. Review.
-
RSL1D1 promotes the progression of colorectal cancer through RAN-mediated autophagy suppression.Cell Death Dis. 2022 Jan 10;13(1):43. doi: 10.1038/s41419-021-04492-z. Cell Death Dis. 2022. PMID: 35013134 Free PMC article.
-
In search of nonribosomal nucleolar protein function and regulation.J Cell Biol. 2009 Mar 23;184(6):771-6. doi: 10.1083/jcb.200812014. Epub 2009 Mar 16. J Cell Biol. 2009. PMID: 19289796 Free PMC article. Review.
References
-
- Baker, S. J. 2007. PTEN enters the nuclear age. Cell 12825-28. - PubMed
-
- Bringold, F., and M. Serrano. 2000. Tumor suppressors and oncogenes in cellular senescence. Exp. Gerontol. 35317-329. - PubMed
-
- Campisi, J. 2001. Cellular senescence as a tumor-suppressor mechanism. Trends Cell Biol. 11S27-S31. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
