Role of secretory phospholipase a(2) in CNS inflammation: implications in traumatic spinal cord injury
- PMID: 18673210
- PMCID: PMC2800081
- DOI: 10.2174/187152708784936671
Role of secretory phospholipase a(2) in CNS inflammation: implications in traumatic spinal cord injury
Abstract
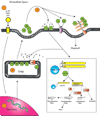
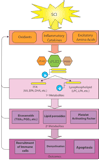
Secretory phospholipases A(2) (sPLA(2)s) are a subfamily of lipolytic enzymes which hydrolyze the acyl bond at the sn-2 position of glycerophospholipids to produce free fatty acids and lysophospholipids. These products are precursors of bioactive eicosanoids and platelet-activating factor (PAF). The hydrolysis of membrane phospholipids by PLA(2) is a rate-limiting step for generation of eicosanoids and PAF. To date, more than 10 isozymes of sPLA(2) have been found in the mammalian central nervous system (CNS). Under physiological conditions, sPLA(2)s are involved in diverse cellular responses, including host defense, phospholipid digestion and metabolism. However, under pathological situations, increased sPLA(2) activity and excessive production of free fatty acids and their metabolites may lead to inflammation, loss of membrane integrity, oxidative stress, and subsequent tissue injury. Emerging evidence suggests that sPLA(2) plays a role in the secondary injury process after traumatic or ischemic injuries in the brain and spinal cord. Importantly, sPLA(2) may act as a convergence molecule that mediates multiple key mechanisms involved in the secondary injury since it can be induced by multiple toxic factors such as inflammatory cytokines, free radicals, and excitatory amino acids, and its activation and metabolites can exacerbate the secondary injury. Blocking sPLA(2) action may represent a novel and efficient strategy to block multiple injury pathways associated with the CNS secondary injury. This review outlines the current knowledge of sPLA(2) in the CNS with emphasis placed on the possible roles of sPLA(2) in mediating CNS injuries, particularly the traumatic and ischemic injuries in the brain and spinal cord.
Figures
Similar articles
-
Phospholipase A2 and its molecular mechanism after spinal cord injury.Mol Neurobiol. 2010 Jun;41(2-3):197-205. doi: 10.1007/s12035-010-8101-0. Epub 2010 Feb 3. Mol Neurobiol. 2010. PMID: 20127525 Free PMC article. Review.
-
Emerging roles of secreted phospholipase A(2) enzymes: an update.Biochimie. 2013 Jan;95(1):43-50. doi: 10.1016/j.biochi.2012.09.007. Epub 2012 Sep 25. Biochimie. 2013. PMID: 23022039 Review.
-
Inflammation, bioactive lipids and atherosclerosis: potential roles of a lipoprotein-associated phospholipase A2, platelet activating factor-acetylhydrolase.Atheroscler Suppl. 2002 Dec;3(4):57-68. doi: 10.1016/s1567-5688(02)00045-4. Atheroscler Suppl. 2002. PMID: 12573364 Review.
-
Expression profile of multiple secretory phospholipase A(2) isoforms in the rat CNS: enriched expression of sPLA(2)-IIA in brainstem and spinal cord.J Chem Neuroanat. 2010 Jul;39(4):242-7. doi: 10.1016/j.jchemneu.2010.02.002. Epub 2010 Feb 11. J Chem Neuroanat. 2010. PMID: 20153419
-
Secreted phospholipase A2, lipoprotein hydrolysis, and atherosclerosis: integration with lipidomics.Anal Bioanal Chem. 2011 Jun;400(7):1829-42. doi: 10.1007/s00216-011-4864-z. Epub 2011 Mar 29. Anal Bioanal Chem. 2011. PMID: 21445663 Free PMC article. Review.
Cited by
-
Regeneration of spinal cord with cell and gene therapy.Orthop Surg. 2009 May;1(2):153-63. doi: 10.1111/j.1757-7861.2009.00018.x. Orthop Surg. 2009. PMID: 22009833 Free PMC article.
-
Phospholipases A2 and inflammatory responses in the central nervous system.Neuromolecular Med. 2010 Jun;12(2):133-48. doi: 10.1007/s12017-009-8092-z. Epub 2009 Oct 24. Neuromolecular Med. 2010. PMID: 19855947 Free PMC article. Review.
-
Delayed LY333013 (Oral) and LY315920 (Intravenous) Reverse Severe Neurotoxicity and Rescue Juvenile Pigs from Lethal Doses of Micrurus fulvius (Eastern Coral Snake) Venom.Toxins (Basel). 2018 Nov 17;10(11):479. doi: 10.3390/toxins10110479. Toxins (Basel). 2018. PMID: 30453607 Free PMC article.
-
Neuroprotection and its molecular mechanism following spinal cord injury.Neural Regen Res. 2012 Sep 15;7(26):2051-62. doi: 10.3969/j.issn.1673-5374.2012.26.007. Neural Regen Res. 2012. PMID: 25624837 Free PMC article. Review.
-
Stability, disposition, and penetration of catalytic antioxidants Mn-porphyrin and Mn-salen and of methylprednisolone in spinal cord injury.Cent Nerv Syst Agents Med Chem. 2012 Jun;12(2):122-30. doi: 10.2174/187152412800792742. Cent Nerv Syst Agents Med Chem. 2012. PMID: 22640221 Free PMC article.
References
-
- Lauber K, Bohn E, Krober SM, Xiao YJ, Blumenthal SG, Lindemann RK, Marini P, Wiedig C, Zobywalski A, Baksh S, Xu Y, Autenrieth IB, Schulze-Osthoff K, Belka C, Stuhler G, Wesselborg S. Cell. 2003;113:717–730. - PubMed
-
- Morell P. Myelin. New York: Plenum Press; 1984.
-
- Schaloske RH, Dennis EA. Biochim. Biophys. Acta. 2006;1761:1246–1259. - PubMed
-
- Murakami M, Nakatani Y, Atsumi G, Inoue K, Kudo I. Crit. Rev. Immunol. 1997;17:225–283. - PubMed
-
- Murakami M, Kudo I. Adv. Immunol. 2001;77:163–194. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P20 RR015576/RR/NCRR NIH HHS/United States
- R01 NS036350-09/NS/NINDS NIH HHS/United States
- R01 NS052290-01/NS/NINDS NIH HHS/United States
- R01 NS036350-03S1/NS/NINDS NIH HHS/United States
- R01 NS036350-02/NS/NINDS NIH HHS/United States
- R01 NS052290-04/NS/NINDS NIH HHS/United States
- R01 NS036350-07/NS/NINDS NIH HHS/United States
- R01 NS052290/NS/NINDS NIH HHS/United States
- R01 NS052290-03/NS/NINDS NIH HHS/United States
- NS52290/NS/NINDS NIH HHS/United States
- R01 NS036350-06A1/NS/NINDS NIH HHS/United States
- NS50253/NS/NINDS NIH HHS/United States
- R01 NS052290-02/NS/NINDS NIH HHS/United States
- R01 NS036350-08/NS/NINDS NIH HHS/United States
- R01 NS052290-05/NS/NINDS NIH HHS/United States
- NS36350/NS/NINDS NIH HHS/United States
- R01 NS036350-10/NS/NINDS NIH HHS/United States
- R01 NS052290-06/NS/NINDS NIH HHS/United States
- F31A5657401/PHS HHS/United States
- R01 NS036350-04/NS/NINDS NIH HHS/United States
- R01 NS111776/NS/NINDS NIH HHS/United States
- R01 NS036350-03/NS/NINDS NIH HHS/United States
- R01 NS036350/NS/NINDS NIH HHS/United States
- R01 NS052290-06S1/NS/NINDS NIH HHS/United States
- RR15576/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
