Regulation of the Warburg effect in early-passage breast cancer cells
- PMID: 18670636
- PMCID: PMC2481565
- DOI: 10.1593/neo.07724
Regulation of the Warburg effect in early-passage breast cancer cells
Abstract
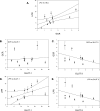
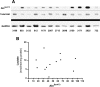
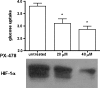
Malignancy in cancer is associated with aerobic glycolysis (Warburg effect) evidenced by increased trapping of [(18)F]deoxyglucose (FdG) in patients imaged by positron emission tomography (PET). [(18)F]deoxyglucose uptake correlates with glucose transporter (GLUT-1) expression, which can be regulated by hypoxia-inducible factor 1 alpha (HIF-1alpha). We have previously reported in established breast lines that HIF-1alpha levels in the presence of oxygen leads to the Warburg effect. However, glycolysis and GLUT-1 can also be induced independent of HIF-1alpha by other factors, such as c-Myc and phosphorylated Akt (pAkt). This study investigates HIF-1alpha, c-Myc, pAkt, and aerobic glycolysis in low-passage breast cancer cells under the assumption that these represent the in vivo condition better than established lines. Similar to in vivo FdG-PET or primary breast cancers, rates of glycolysis were diverse, being higher in cells expressing both c-Myc and HIF-1alpha and lower in cell lines low or negative in both transcription factors. No correlations were observed between glycolytic rates and pAkt levels. Two of 12 cell lines formed xenografts in mice. Both were positive for HIF-1alpha and phosphorylated c-Myc, and only one was positive for pAkt. Glycolysis was affected by pharmacological regulation of c-Myc and HIF-1alpha. These findings suggest that c-Myc and/or HIF-1alpha activities are both involved in the regulation of glycolysis in breast cancers.
Figures



Similar articles
-
Resveratrol suppresses cancer cell glucose uptake by targeting reactive oxygen species-mediated hypoxia-inducible factor-1α activation.J Nucl Med. 2013 Dec;54(12):2161-7. doi: 10.2967/jnumed.112.115436. Epub 2013 Nov 12. J Nucl Med. 2013. PMID: 24221993
-
Early changes in [18F]FDG incorporation by breast cancer cells treated with trastuzumab in normoxic conditions: role of the Akt-pathway, glucose transport and HIF-1α.Breast Cancer Res Treat. 2014 Apr;144(2):241-8. doi: 10.1007/s10549-014-2858-1. Epub 2014 Feb 13. Breast Cancer Res Treat. 2014. PMID: 24522376
-
In vivo evaluation of the effects of simultaneous inhibition of GLUT-1 and HIF-1α by antisense oligodeoxynucleotides on the radiosensitivity of laryngeal carcinoma using micro 18F-FDG PET/CT.Oncotarget. 2017 May 23;8(21):34709-34726. doi: 10.18632/oncotarget.16671. Oncotarget. 2017. PMID: 28410229 Free PMC article.
-
Metabolic phenotype of bladder cancer.Cancer Treat Rev. 2016 Apr;45:46-57. doi: 10.1016/j.ctrv.2016.03.005. Epub 2016 Mar 8. Cancer Treat Rev. 2016. PMID: 26975021 Review.
-
How does hypoxia inducible factor-1α participate in enhancing the glycolysis activity in cervical cancer?Ann Diagn Pathol. 2013 Jun;17(3):305-11. doi: 10.1016/j.anndiagpath.2012.12.002. Epub 2013 Feb 1. Ann Diagn Pathol. 2013. PMID: 23375385 Review.
Cited by
-
Progressive increase of glucose transporter-3 (GLUT-3) expression in estrogen-induced breast carcinogenesis.Clin Transl Oncol. 2013 Jan;15(1):55-64. doi: 10.1007/s12094-012-0882-3. Epub 2012 Oct 2. Clin Transl Oncol. 2013. PMID: 23054751 Free PMC article.
-
SWATH-MS based quantitative proteomics analysis reveals that curcumin alters the metabolic enzyme profile of CML cells by affecting the activity of miR-22/IPO7/HIF-1α axis.J Exp Clin Cancer Res. 2018 Jul 25;37(1):170. doi: 10.1186/s13046-018-0843-y. J Exp Clin Cancer Res. 2018. PMID: 30045750 Free PMC article.
-
SIRT3 elicited an anti-Warburg effect through HIF1α/PDK1/PDHA1 to inhibit cholangiocarcinoma tumorigenesis.Cancer Med. 2019 May;8(5):2380-2391. doi: 10.1002/cam4.2089. Epub 2019 Apr 16. Cancer Med. 2019. PMID: 30993888 Free PMC article.
-
Altered glucose metabolism in Harvey-ras transformed MCF10A cells.Mol Carcinog. 2015 Feb;54(2):111-20. doi: 10.1002/mc.22079. Epub 2013 Sep 2. Mol Carcinog. 2015. PMID: 24000146 Free PMC article.
-
Oxygen sensing, mitochondrial biology and experimental therapeutics for pulmonary hypertension and cancer.Free Radic Biol Med. 2021 Jul;170:150-178. doi: 10.1016/j.freeradbiomed.2020.12.452. Epub 2021 Jan 12. Free Radic Biol Med. 2021. PMID: 33450375 Free PMC article.
References
-
- Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, Feuer EJ, Thun MJ. Cancer statistics, 2005. CA Cancer J Clin. 2005;55(1):10–30. - PubMed
-
- Kumar R, Alavi A. Fluorodeoxyglucose-PET in the management of breast cancer. Radiol Clin North Am. 2004;42(6):1113–1122. ix. - PubMed
-
- Kumar R, Zhuang H, Schnall M, Conant E, Damia S, Weinstein S, Chandra P, Czerniecki B, Alavi A. FDG PET positive lymph nodes are highly predictive of metastasis in breast cancer. Nucl Med Commun. 2006;27(3):231–236. - PubMed
-
- Moon DH, Maddahi J, Silverman DH, Glaspy JA, Phelps ME, Hoh CK. Accuracy of whole-body fluorine-18-FDG PET for the detection of recurrent or metastatic breast carcinoma. J Nucl Med. 1998;39(3):431–435. - PubMed
-
- Oshida M, Uno K, Suzuki M, Nagashima T, Hashimoto H, Yagata H, Shishikura T, Imazeki K, Nakajima N. Predicting the prognoses of breast carcinoma patients with positron emission tomography using 2-deoxy-2-fluoro[18F]-d-glucose. Cancer. 1998;82(11):2227–2234. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
