Physiology, structure, and regulation of the cloned organic anion transporters
- PMID: 18668434
- PMCID: PMC2644066
- DOI: 10.1080/00498250801927435
Physiology, structure, and regulation of the cloned organic anion transporters
Abstract
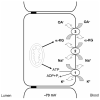
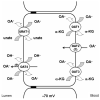
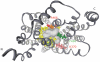
1. The transport of negatively charged drugs, xenobiotics, and metabolites by epithelial tissues, particularly the kidney, plays critical roles in controlling their distribution, concentration, and retention in the body. Thus, organic anion transporters (OATs) impact both their therapeutic efficacy and potential toxicity. 2. This review summarizes current knowledge of the properties and functional roles of the cloned OATs, the relationships between transporter structure and function, and those factors that determine the efficacy of transport. Such factors include plasma protein binding of substrates, genetic polymorphisms among the transporters, and regulation of transporter expression. 3. Clearly, much progress has been made in the decade since the first OAT was cloned. However, unresolved questions remain. Several of these issues--drug-drug interactions, functional characterization of newly cloned OATs, tissue differences in expression and function, and details of the nature and consequences of transporter regulation at genomic and intracellular sites--are discussed in the concluding Perspectives section.
Figures
Similar articles
-
Update on the molecular physiology of organic anion transporters.Curr Opin Nephrol Hypertens. 2008 Sep;17(5):499-505. doi: 10.1097/MNH.0b013e32830b5d5d. Curr Opin Nephrol Hypertens. 2008. PMID: 18695391 Review.
-
Structure, function, and regulation of renal organic anion transporters.Med Res Rev. 2002 Nov;22(6):602-16. doi: 10.1002/med.10019. Med Res Rev. 2002. PMID: 12369090 Review.
-
Organic anion transporters: discovery, pharmacology, regulation and roles in pathophysiology.Biopharm Drug Dispos. 2010 Jan;31(1):1-71. doi: 10.1002/bdd.693. Biopharm Drug Dispos. 2010. PMID: 19953504 Review.
-
Renal organic anion transporters (SLC22 family): expression, regulation, roles in toxicity, and impact on injury and disease.AAPS J. 2013 Jan;15(1):53-69. doi: 10.1208/s12248-012-9413-y. Epub 2012 Oct 9. AAPS J. 2013. PMID: 23054972 Free PMC article. Review.
-
[Progress in study on organic anion transporters].Sheng Li Ke Xue Jin Zhan. 2012 Feb;43(1):11-6. Sheng Li Ke Xue Jin Zhan. 2012. PMID: 22582592 Review. Chinese.
Cited by
-
Membrane Carriers and Transporters in Kidney Physiology and Disease.Biomedicines. 2021 Apr 14;9(4):426. doi: 10.3390/biomedicines9040426. Biomedicines. 2021. PMID: 33919957 Free PMC article. Review.
-
P2' benzene carboxylic acid moiety is associated with decrease in cellular uptake: evaluation of novel nonpeptidic HIV-1 protease inhibitors containing P2 bis-tetrahydrofuran moiety.Antimicrob Agents Chemother. 2013 Oct;57(10):4920-7. doi: 10.1128/AAC.00868-13. Epub 2013 Jul 22. Antimicrob Agents Chemother. 2013. PMID: 23877703 Free PMC article.
-
Posttranslational Regulation of Organic Anion Transporters by Ubiquitination: Known and Novel.Med Res Rev. 2016 Sep;36(5):964-79. doi: 10.1002/med.21397. Epub 2016 Jun 12. Med Res Rev. 2016. PMID: 27291023 Free PMC article. Review.
-
Drug uptake systems in liver and kidney: a historic perspective.Clin Pharmacol Ther. 2010 Jan;87(1):39-47. doi: 10.1038/clpt.2009.235. Epub 2009 Nov 18. Clin Pharmacol Ther. 2010. PMID: 19924123 Free PMC article. Review.
-
Regulation of human organic anion transporter 4 by protein kinase C and NHERF-1: altering the endocytosis of the transporter.Pharm Res. 2010 Apr;27(4):589-96. doi: 10.1007/s11095-009-9983-2. Epub 2010 Feb 6. Pharm Res. 2010. PMID: 20140636 Free PMC article.
References
-
- Abramson J, Smirnova I, Kasho V, Verner G, Iwata S, Kaback HR. The lactose permease of Escherichia coli: Overall structure, the sugar-binding site and the alternating access model for transport. Federation of European Biochemical Societies Letters. 2003a;555:96–101. - PubMed
-
- Abramson J, Smirnova I, Kasho V, Verner G, Kaback HR, Iwata S. Structure and mechanism of the lactose permease of Escherichia coli. Science. 2003b;301(5633):610–615. - PubMed
-
- Ait Slimane T, Hoekstra D. Sphingolipid trafficking and protein sorting in epithelial cells. Federation of European Biochemical Societies Letters. 2002;529:54–59. - PubMed
-
- Anzai N, Jutabha P, Enomoto A, Yokoyama H, Nonoguchi H, Hirata T, Shiraya K, He X, Cha SH, Takeda M, et al. Functional characterization of rat organic anion transporter 5 (Slc22a19) at the apical membrane of renal proximal tubules. Journal of Pharmacology and Experimental Therapeutics. 2005;315:534–544. - PubMed
-
- Anzai N, Kanai Y, Endou H. Organic anion transporter family: Current knowledge. Journal of Pharmacological Sciences. 2006;100:411–426. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
