Caveolin-1 regulates human immunodeficiency virus-1 Tat-induced alterations of tight junction protein expression via modulation of the Ras signaling
- PMID: 18667611
- PMCID: PMC2635104
- DOI: 10.1523/JNEUROSCI.0061-08.2008
Caveolin-1 regulates human immunodeficiency virus-1 Tat-induced alterations of tight junction protein expression via modulation of the Ras signaling
Abstract
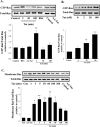
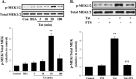
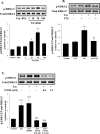
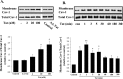
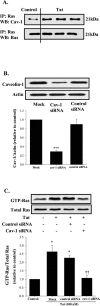
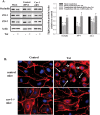
The blood-brain barrier (BBB) is the critical structure for preventing human immunodeficiency virus (HIV) trafficking into the brain. Specific HIV proteins, such as Tat protein, can contribute to the dysfunction of tight junctions at the BBB and HIV entry into the brain. Tat is released by HIV-1-infected cells and can interact with a variety of cell surface receptors activating several signal transduction pathways, including those localized in caveolae. The present study focused on the mechanisms of Tat-induced caveolae-associated Ras signaling at the level of the BBB. Treatment with Tat activated the Ras pathway in human brain microvascular endothelial cells (HBMECs). However, caveolin-1 silencing markedly attenuated these effects. Because the integrity of the brain endothelium is regulated by intercellular tight junctions, these structural elements of the BBB were also evaluated in the present study. Exposure to Tat diminished the expression of several tight junction proteins, namely, occludin, zonula occludens (ZO)-1, and ZO-2 in the caveolar fraction of HBMECs. These effects were effectively protected by pharmacological inhibition of the Ras signaling and by silencing of caveolin-1. The present data indicate the importance of caveolae-associated signaling in the disruption of tight junctions on Tat exposure. They also demonstrate that caveolin-1 may constitute an early and critical modulator that controls signaling pathways leading to the disruption of tight junction proteins. Thus, caveolin-1 may provide an effective target to protect against Tat-induced HBMEC dysfunction and the disruption of the BBB in HIV-1-infected patients.
Figures

Similar articles
-
HIV-1 Tat protein alters tight junction protein expression and distribution in cultured brain endothelial cells.J Neurosci Res. 2003 Oct 15;74(2):255-65. doi: 10.1002/jnr.10762. J Neurosci Res. 2003. PMID: 14515355
-
HIV-1 Tat triggers nuclear localization of ZO-1 via Rho signaling and cAMP response element-binding protein activation.J Neurosci. 2012 Jan 4;32(1):143-50. doi: 10.1523/JNEUROSCI.4266-11.2012. J Neurosci. 2012. PMID: 22219277 Free PMC article.
-
Limited role of COX-2 in HIV Tat-induced alterations of tight junction protein expression and disruption of the blood-brain barrier.Brain Res. 2007 Dec 12;1184:333-44. doi: 10.1016/j.brainres.2007.09.063. Epub 2007 Oct 2. Brain Res. 2007. PMID: 17976544
-
Zonula occludens (ZO)-1 and ZO-2: membrane-associated guanylate kinase homologues (MAGuKs) of the tight junction.Biochem Soc Trans. 1995 Aug;23(3):470-5. doi: 10.1042/bst0230470. Biochem Soc Trans. 1995. PMID: 8566365 Review. No abstract available.
-
Disruption of blood-brain barrier: effects of HIV Tat on brain microvascular endothelial cells and tight junction proteins.J Neurovirol. 2023 Dec;29(6):658-668. doi: 10.1007/s13365-023-01179-3. Epub 2023 Oct 29. J Neurovirol. 2023. PMID: 37899420 Review.
Cited by
-
Time-dependent dual effects of high levels of unconjugated bilirubin on the human blood-brain barrier lining.Front Cell Neurosci. 2012 May 10;6:22. doi: 10.3389/fncel.2012.00022. eCollection 2012. Front Cell Neurosci. 2012. PMID: 22590454 Free PMC article.
-
Occludin, caveolin-1, and Alix form a multi-protein complex and regulate HIV-1 infection of brain pericytes.FASEB J. 2020 Dec;34(12):16319-16332. doi: 10.1096/fj.202001562R. Epub 2020 Oct 14. FASEB J. 2020. PMID: 33058236 Free PMC article.
-
Temporal Requirements of cMyc Protein for Reprogramming Mouse Fibroblasts.Stem Cells Int. 2012;2012:541014. doi: 10.1155/2012/541014. Epub 2012 Apr 26. Stem Cells Int. 2012. PMID: 22619682 Free PMC article.
-
Polychlorinated biphenyls disrupt blood-brain barrier integrity and promote brain metastasis formation.Environ Health Perspect. 2010 Apr;118(4):479-84. doi: 10.1289/ehp.0901334. Epub 2009 Oct 28. Environ Health Perspect. 2010. PMID: 20064788 Free PMC article.
-
Human immunodeficiency virus-1 Tat activates calpain proteases via the ryanodine receptor to enhance surface dopamine transporter levels and increase transporter-specific uptake and Vmax.J Neurosci. 2010 Oct 20;30(42):14153-64. doi: 10.1523/JNEUROSCI.1042-10.2010. J Neurosci. 2010. PMID: 20962236 Free PMC article.
References
-
- Abbott NJ, Rönnbäck L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. - PubMed
-
- Albini A, Soldi R, Giunciuglio D, Giraudo E, Benelli R, Primo L, Noonan D, Salio M, Camussi G, Rockl W, Bussolino F. The angiogenesis induced by HIV-1 tat protein is mediated by the Flk-1/KDR receptor on vascular endothelial cells. Nat Med. 1996;2:1371–1375. - PubMed
-
- András IE, Pu H, Deli MA, Nath A, Hennig B, Toborek M. HIV-1 Tat protein alters tight junction protein expression and distribution in cultured brain endothelial cells. J Neurosci Res. 2003;74:255–265. - PubMed
-
- András IE, Pu H, Tian J, Deli MA, Nath A, Hennig B, Toborek M. Signaling mechanisms of HIV-1 Tat-induced alterations of claudin-5 expression in brain endothelial cells. J Cereb Blood Flow Metab. 2005;25:1159–1170. - PubMed
-
- Annunziata P. Blood-brain barrier changes during invasion of the central nervous system by HIV-1. Old and new insights into the mechanism. J Neurol. 2003;250:901–906. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 MH063022/MH/NIMH NIH HHS/United States
- MH063022/MH/NIMH NIH HHS/United States
- R01 MH063022-05/MH/NIMH NIH HHS/United States
- P01 DA019398/DA/NIDA NIH HHS/United States
- P42 ES007380/ES/NIEHS NIH HHS/United States
- R01 DA027569/DA/NIDA NIH HHS/United States
- NS39254/NS/NINDS NIH HHS/United States
- P42 ES007380-120009/ES/NIEHS NIH HHS/United States
- P01 DA19398/DA/NIDA NIH HHS/United States
- R01 MH063022-06A2/MH/NIMH NIH HHS/United States
- R01 MH072567/MH/NIMH NIH HHS/United States
- R01 MH072567-04/MH/NIMH NIH HHS/United States
- R01 NS039254/NS/NINDS NIH HHS/United States
- MH072567/MH/NIMH NIH HHS/United States
- R01 MH098891/MH/NIMH NIH HHS/United States
- R01 NS039254-09/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

