Association of the cellular coactivator HCF-1 with the Golgi apparatus in sensory neurons
- PMID: 18667495
- PMCID: PMC2546983
- DOI: 10.1128/JVI.01174-08
Association of the cellular coactivator HCF-1 with the Golgi apparatus in sensory neurons
Abstract
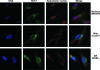
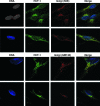
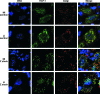
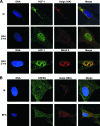
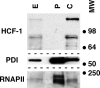
HCF-1 is a cellular transcriptional coactivator that is critical for mediating the regulated expression of the immediate-early genes of the alphaherpesviruses herpes simplex virus type 1 and varicella-zoster virus. HCF-1 functions, at least in part, by modulating the modification of nucleosomes at these viral promoters to reverse cell-mediated repressive marks and promote activating marks. Strikingly, HCF-1 is specifically sequestered in the cytoplasm of sensory neurons where these viruses establish latency and is rapidly relocalized to the nucleus upon stimuli that result in viral reactivation. However, the analysis of HCF-1 in latently infected neurons and the protein's specific subcellular location have not been determined. Therefore, in this study, the localization of HCF-1 in unstimulated and induced latently infected sensory neurons was investigated and was found to be similar to that observed in uninfected mice, with a time course of induced nuclear accumulation that correlated with viral reactivation. Using a primary neuronal cell culture system, HCF-1 was localized to the Golgi apparatus in unstimulated neurons, a unique location for a transcriptional coactivator. Upon disruption of the Golgi body, HCF-1 was rapidly relocalized to the nucleus in contrast to other Golgi apparatus-associated proteins. The location of HCF-1 is distinct from that of CREB3, an endoplasmic reticulum-resident HCF-1 interaction partner that has been proposed to sequester HCF-1. The results support the model that HCF-1 is an important component of the viral latency-reactivation cycle and that it is regulated by association with a component that is distinct from the identified HCF-1 interaction factors.
Figures

Similar articles
-
Recruitment of the transcriptional coactivator HCF-1 to viral immediate-early promoters during initiation of reactivation from latency of herpes simplex virus type 1.J Virol. 2009 Sep;83(18):9591-5. doi: 10.1128/JVI.01115-09. Epub 2009 Jul 1. J Virol. 2009. PMID: 19570863 Free PMC article.
-
The Cellular Coactivator HCF-1 Is Required for Glucocorticoid Receptor-Mediated Transcription of Bovine Herpesvirus 1 Immediate Early Genes.J Virol. 2018 Aug 16;92(17):e00987-18. doi: 10.1128/JVI.00987-18. Print 2018 Sep 1. J Virol. 2018. PMID: 29899098 Free PMC article.
-
The coactivator host cell factor-1 mediates Set1 and MLL1 H3K4 trimethylation at herpesvirus immediate early promoters for initiation of infection.Proc Natl Acad Sci U S A. 2007 Jun 26;104(26):10835-40. doi: 10.1073/pnas.0704351104. Epub 2007 Jun 19. Proc Natl Acad Sci U S A. 2007. PMID: 17578910 Free PMC article.
-
Control of alpha-herpesvirus IE gene expression by HCF-1 coupled chromatin modification activities.Biochim Biophys Acta. 2010 Mar-Apr;1799(3-4):257-65. doi: 10.1016/j.bbagrm.2009.08.003. Epub 2009 Aug 12. Biochim Biophys Acta. 2010. PMID: 19682612 Free PMC article. Review.
-
The dynamics of HCF-1 modulation of herpes simplex virus chromatin during initiation of infection.Viruses. 2013 May 22;5(5):1272-91. doi: 10.3390/v5051272. Viruses. 2013. PMID: 23698399 Free PMC article. Review.
Cited by
-
Roles of the nuclear lamina in stable nuclear association and assembly of a herpesviral transactivator complex on viral immediate-early genes.mBio. 2012 Jan 17;3(1):e00300-11. doi: 10.1128/mBio.00300-11. Print 2012. mBio. 2012. PMID: 22251972 Free PMC article.
-
Targeting the JMJD2 histone demethylases to epigenetically control herpesvirus infection and reactivation from latency.Sci Transl Med. 2013 Jan 9;5(167):167ra5. doi: 10.1126/scitranslmed.3005145. Sci Transl Med. 2013. PMID: 23303604 Free PMC article.
-
[Mechanisms of herpes simplex virus latency and reactivation].Zhejiang Da Xue Xue Bao Yi Xue Ban. 2019 May 25;48(1):89-101. doi: 10.3785/j.issn.1008-9292.2019.02.14. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2019. PMID: 31102363 Free PMC article. Review. Chinese.
-
Models of Herpes Simplex Virus Latency.Viruses. 2024 May 8;16(5):747. doi: 10.3390/v16050747. Viruses. 2024. PMID: 38793628 Free PMC article. Review.
-
Herpes Simplex Virus Establishment, Maintenance, and Reactivation: In Vitro Modeling of Latency.Pathogens. 2017 Jun 23;6(3):28. doi: 10.3390/pathogens6030028. Pathogens. 2017. PMID: 28644417 Free PMC article. Review.
References
-
- Amelio, A. L., N. V. Giordani, N. J. Kubat, J. E. O'Neil, and D. C. Bloom. 2006. Deacetylation of the herpes simplex virus type 1 latency-associated transcript (LAT) enhancer and a decrease in LAT abundance precede an increase in ICP0 transcriptional permissiveness at early times postexplant. J. Virol. 802063-2068. - PMC - PubMed
-
- Bailey, D., and P. O'Hare. 2007. Transmembrane bZIP transcription factors in ER stress signaling and the unfolded protein response. Antioxid. Redox Signal. 92305-2321. - PubMed
-
- Brown, M. S., and J. L. Goldstein. 1997. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell 89331-340. - PubMed
-
- Chen, X., J. Shen, and R. Prywes. 2002. The luminal domain of ATF6 senses endoplasmic reticulum (ER) stress and causes translocation of ATF6 from the ER to the Golgi. J. Biol. Chem. 27713045-13052. - PubMed
-
- Delehouzee, S., T. Yoshikawa, C. Sawa, J. Sawada, T. Ito, M. Omori, T. Wada, Y. Yamaguchi, Y. Kabe, and H. Handa. 2005. GABP, HCF-1 and YY1 are involved in Rb gene expression during myogenesis. Genes Cells 10717-731. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

