A leaky blood-brain barrier, fibrinogen infiltration and microglial reactivity in inflamed Alzheimer's disease brain
- PMID: 18657226
- PMCID: PMC4498946
- DOI: 10.1111/j.1582-4934.2008.00434.x
A leaky blood-brain barrier, fibrinogen infiltration and microglial reactivity in inflamed Alzheimer's disease brain
Abstract
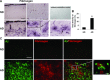
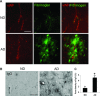
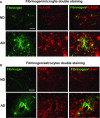
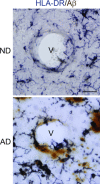
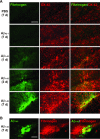
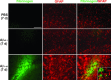
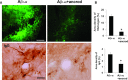
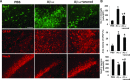
This study has used immunohistochemical examination of tissue obtained from Alzheimer's disease (AD) brains and rat hippocampus injected with Abeta(1-42) peptide to determine effects of induced inflammatory reactivity on integrity of blood-brain barrier (BBB) and viability of neurons. Tissue from AD, but not non-demented, brains exhibited a diffuse pattern of staining for fibrinogen and immunoglobulin (IgG) indicative of BBB leakiness with considerable fibrinogen immunoreactivity (ir) appearing in association with Abeta deposits. Immunostaining for the endothelial cell specific glycoprotein, von Willebrand factor, showed morphological evidence for altered blood vessels in AD tissue. AD brains also demonstrated extensive areas of fibrinogen ir in association with microglial reactivity. In vivo, intra-hippocampal injection of Abeta(1-42) caused time-dependent (1-7 days after injection) increases in double staining of fibrinogen with areas of microgliosis. Two independent pharmacological strategies were employed to examine how Abeta(1-42) stimulation (7 days injection) may be linked to neurodegeneration. The defibrinogenating compound, ancrod, reduced inflammatory reactivity, levels of parenchymal fibrinogen and IgG, and was neuroprotective. These results prompted use of Abeta(1-42) plus fibrinogen as a novel in vivo inflammatory stimulus and this combination significantly enhanced inflammatory reactivity, vascular perturbations and neuronal damage compared to Abeta(1-42) alone. A second approach, using anti-Mac-1 (antibody for antigen CD11b) to block activation of microglia, was highly effective in attenuating effects of Abeta(1-42) plus fibrinogen amplification of inflammatory and vascular responses and conferred significant neuroprotection. The overall findings from study of AD tissue and in vivo in Abeta(1-42) and Abeta(1-42) plus fibrinogen stimulated rat hippocampus suggest microglial responses to promote increased extravasation of blood protein as a critical component in amplifying inflammatory reactivity and causing neuronal damage in inflamed AD brain.
Figures

Similar articles
-
VEGF receptor antagonist Cyclo-VEGI reduces inflammatory reactivity and vascular leakiness and is neuroprotective against acute excitotoxic striatal insult.J Neuroinflammation. 2008 May 20;5:18. doi: 10.1186/1742-2094-5-18. J Neuroinflammation. 2008. PMID: 18492281 Free PMC article.
-
Pharmacological antagonism of interleukin-8 receptor CXCR2 inhibits inflammatory reactivity and is neuroprotective in an animal model of Alzheimer's disease.J Neuroinflammation. 2015 Aug 9;12:144. doi: 10.1186/s12974-015-0339-z. J Neuroinflammation. 2015. PMID: 26255110 Free PMC article.
-
Microglial VEGF receptor response is an integral chemotactic component in Alzheimer's disease pathology.J Neurosci. 2009 Jan 7;29(1):3-13. doi: 10.1523/JNEUROSCI.2888-08.2009. J Neurosci. 2009. PMID: 19129379 Free PMC article.
-
A Leaky Blood-Brain Barrier to Fibrinogen Contributes to Oxidative Damage in Alzheimer's Disease.Antioxidants (Basel). 2021 Dec 31;11(1):102. doi: 10.3390/antiox11010102. Antioxidants (Basel). 2021. PMID: 35052606 Free PMC article. Review.
-
Fibrinogen and altered hemostasis in Alzheimer's disease.J Alzheimers Dis. 2012;32(3):599-608. doi: 10.3233/JAD-2012-120820. J Alzheimers Dis. 2012. PMID: 22869464 Free PMC article. Review.
Cited by
-
Blood protein predictors of brain amyloid for enrichment in clinical trials?Alzheimers Dement (Amst). 2015 Mar 29;1(1):48-60. doi: 10.1016/j.dadm.2014.11.005. eCollection 2015 Mar. Alzheimers Dement (Amst). 2015. PMID: 27239491 Free PMC article.
-
T Cells Actively Infiltrate the White Matter of the Aging Monkey Brain in Relation to Increased Microglial Reactivity and Cognitive Decline.Front Immunol. 2021 Feb 16;12:607691. doi: 10.3389/fimmu.2021.607691. eCollection 2021. Front Immunol. 2021. PMID: 33664743 Free PMC article.
-
Melatonin ameliorates microvessel abnormalities in the cerebral cortex and hippocampus in a rat model of Alzheimer's disease.Neural Regen Res. 2021 Apr;16(4):757-764. doi: 10.4103/1673-5374.295349. Neural Regen Res. 2021. PMID: 33063739 Free PMC article.
-
The APOE ɛ4/ɛ4 genotype potentiates vascular fibrin(ogen) deposition in amyloid-laden vessels in the brains of Alzheimer's disease patients.J Cereb Blood Flow Metab. 2013 Aug;33(8):1251-8. doi: 10.1038/jcbfm.2013.76. Epub 2013 May 8. J Cereb Blood Flow Metab. 2013. PMID: 23652625 Free PMC article.
-
Lecanemab Blocks the Effects of the Aβ/Fibrinogen Complex on Blood Clots and Synapse Toxicity in Organotypic Culture.bioRxiv [Preprint]. 2024 Jan 21:2024.01.20.576458. doi: 10.1101/2024.01.20.576458. bioRxiv. 2024. Update in: Proc Natl Acad Sci U S A. 2024 Apr 23;121(17):e2314450121. doi: 10.1073/pnas.2314450121 PMID: 38293058 Free PMC article. Updated. Preprint.
References
-
- Kalaria RN. The blood-brain barrier and cerebrovascular pathology in Alzheimer’s disease. Ann N Y Acad Sci. 1999;893:113–25. - PubMed
-
- Kalaria RN. Small vessel disease and Alzheimer’s dementia: pathological considerations. Cerebrovasc Dis. 2002;13:48–52. - PubMed
-
- De La Torre JC. Is Alzheimer’s disease a neurodegenerative or a vascular disorder? Data, dogma, and dialectics. Lancet Neurol. 2004;3:184–90. - PubMed
-
- Zipser BD, Johanson CE, Gonzalez L, et al. Microvascular injury and blood-brain barrier leakage in Alzheimer’s disease. Neurobiol Aging. 2007;28:977–86. - PubMed
-
- Tomimoto H, Akiguchi I, Suenaga T, et al. Alterations of the blood-brain barrier and glial cells in white-matter lesions in cerebrovascular and Alzheimer’s disease patients. Stroke. 1996;27:2069–74. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

