Deciphering downstream gene targets of PI3K/mTOR/p70S6K pathway in breast cancer
- PMID: 18652687
- PMCID: PMC2496917
- DOI: 10.1186/1471-2164-9-348
Deciphering downstream gene targets of PI3K/mTOR/p70S6K pathway in breast cancer
Abstract
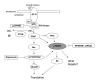
Background: The 70 kDa ribosomal protein S6 kinase (RPS6KB1), located at 17q23, is amplified and overexpressed in 10-30% of primary breast cancers and breast cancer cell lines. p70S6K is a serine/threonine kinase regulated by PI3K/mTOR pathway, which plays a crucial role in control of cell cycle, growth and survival. Our aim was to determine p70S6K and PI3K/mTOR/p70S6K pathway dependent gene expression profiles by microarrays using five breast cancer cell lines with predefined gene copy number and gene expression alterations. The p70S6K dependent profiles were determined by siRNA silencing of RPS6KB1 in two breast cancer cell lines overexpressing p70S6K. These profiles were further correlated with gene expression alterations caused by inhibition of PI3K/mTOR pathway with PI3K inhibitor Ly294002 or mTOR inhibitor rapamycin.
Results: Altogether, the silencing of p70S6K altered the expression of 109 and 173 genes in two breast cancer cell lines and 67 genes were altered in both cell lines in addition to RPS6KB1. Furthermore, 17 genes including VTCN1 and CDKN2B showed overlap with genes differentially expressed after PI3K or mTOR inhibition. The gene expression signatures responsive to both PI3K/mTOR pathway and p70S6K inhibitions revealed previously unidentified genes suggesting novel downstream targets for PI3K/mTOR/p70S6K pathway.
Conclusion: Since p70S6K overexpression is associated with aggressive disease and poor prognosis of breast cancer patients, the potential downstream targets of p70S6K and the whole PI3K/mTOR/p70S6K pathway identified in our study may have diagnostic value.
Figures
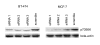
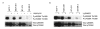
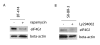
Similar articles
-
Phospho-p70S6K/p85S6K and cdc2/cdk1 are novel targets for diffuse large B-cell lymphoma combination therapy.Clin Cancer Res. 2009 Mar 1;15(5):1708-20. doi: 10.1158/1078-0432.CCR-08-1543. Epub 2009 Feb 17. Clin Cancer Res. 2009. PMID: 19223503
-
Activation of mammalian target of rapamycin signaling pathway contributes to tumor cell survival in anaplastic lymphoma kinase-positive anaplastic large cell lymphoma.Cancer Res. 2006 Jul 1;66(13):6589-97. doi: 10.1158/0008-5472.CAN-05-3018. Cancer Res. 2006. PMID: 16818631 Free PMC article.
-
Prolactin activates mammalian target-of-rapamycin through phosphatidylinositol 3-kinase and stimulates phosphorylation of p70S6K and 4E-binding protein-1 in lymphoma cells.J Endocrinol. 2006 Aug;190(2):307-12. doi: 10.1677/joe.1.06368. J Endocrinol. 2006. PMID: 16899564
-
New targets for therapy in breast cancer: mammalian target of rapamycin (mTOR) antagonists.Breast Cancer Res. 2004;6(5):219-24. doi: 10.1186/bcr927. Epub 2004 Aug 12. Breast Cancer Res. 2004. PMID: 15318929 Free PMC article. Review.
-
The emerging role of mammalian target of rapamycin inhibitors in the treatment of sarcomas.Target Oncol. 2011 Mar;6(1):29-39. doi: 10.1007/s11523-011-0179-4. Epub 2011 Apr 28. Target Oncol. 2011. PMID: 21533543 Review.
Cited by
-
Poly (A)+ transcriptome assessment of ERBB2-induced alterations in breast cell lines.PLoS One. 2011;6(6):e21022. doi: 10.1371/journal.pone.0021022. Epub 2011 Jun 22. PLoS One. 2011. PMID: 21731642 Free PMC article.
-
20(S)-Ginsenoside Rh2 displays efficacy against T-cell acute lymphoblastic leukemia through the PI3K/Akt/mTOR signal pathway.J Ginseng Res. 2020 Sep;44(5):725-737. doi: 10.1016/j.jgr.2019.07.003. Epub 2019 Jul 30. J Ginseng Res. 2020. PMID: 32913402 Free PMC article.
-
TCTP is an androgen-regulated gene implicated in prostate cancer.PLoS One. 2013 Jul 22;8(7):e69398. doi: 10.1371/journal.pone.0069398. Print 2013. PLoS One. 2013. PMID: 23894469 Free PMC article.
-
Poly(adenosine diphosphate ribose) polymerase inhibitors induce autophagy-mediated drug resistance in ovarian cancer cells, xenografts, and patient-derived xenograft models.Cancer. 2020 Feb 15;126(4):894-907. doi: 10.1002/cncr.32600. Epub 2019 Nov 12. Cancer. 2020. PMID: 31714594 Free PMC article.
-
Prucalopride inhibits the glioma cells proliferation and induces autophagy via AKT-mTOR pathway.BMC Neurol. 2018 Jun 4;18(1):80. doi: 10.1186/s12883-018-1083-7. BMC Neurol. 2018. PMID: 29866060 Free PMC article.
References
-
- Couch FJ, Wang XY, Wu GJ, Qian J, Jenkins RB, James CD. Localization of PS6K to Chromosomal Region 17q23 and Determination of its Amplification in Breast Cancer. Cancer Res. 1999;59:1408–1411. - PubMed
-
- Bärlund M, Forozan F, Kononen J, Bubendorf L, Chen Y, Bittner ML, Torhorst J, Haas P, Bucher C, Sauter G, Kallioniemi OP, Kallioniemi A. Detecting Activation of Ribosomal Protein S6 Kinase by Complementary DNA and Tissue Microarray Analysis. J Natl Cancer Inst. 2000;92:1252–1259. doi: 10.1093/jnci/92.15.1252. - DOI - PubMed
-
- Bärlund M, Monni O, Kononen J, Cornelison R, Torhorst J, Sauter G, Kallioniemi OP, Kallioniemi A. Multiple genes at 17q23 undergo amplification and overexpression in breast cancer. Cancer Res. 2000;60:5340–5344. - PubMed
-
- Monni O, Bärlund M, Mousses S, Kononen J, Sauter G, Heiskanen M, Paavola P, Avela K, Chen Y, Bittner ML, Kallioniemi A. Comprehensive copy number and gene expression profiling of the 17q23 amplicon in human breast cancer. Proc Natl Acad Sci USA. 2001;98:5711–5716. doi: 10.1073/pnas.091582298. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

