Apoptotic peptides derived from HIV-1 Nef induce lymphocyte depletion in mice
- PMID: 18646317
- PMCID: PMC3218084
Apoptotic peptides derived from HIV-1 Nef induce lymphocyte depletion in mice
Abstract
Introduction: We have developed a mouse model to examine the effects of host exposure (ie, hematopoietic system) to secreted HIV-1 Nef or peptides derived from Nef.
Methods: We used a combination of terminal uridine deoxynucleotidyl transferase (dUTP) nick end labeling (TUNEL) assays and CD4+ cell counts to assess the status of circulating immune cells in mice treated with Nef-derived proteins.
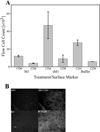
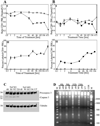
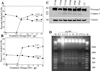
Results: Mice treated with peptides derived from HIV-1 Nef protein displayed significant increases in apoptotic CD4+ lymphocytes and thymus cells and significant decreases in the numbers of circulating CD4+ lymphocytes. No effects were observed in mice treated with controls. There was a clear dose- and time-response relationship between cell changes and the amount of protein or peptide. induction of multiple markers of apoptosis such as DNA laddering and caspase 3 activation was observed during dose- or time-response experiments. Cell death and lymphocyte depletion were blocked by induction of a humoral response to the HIV Nef apoptotic epitope.
Conclusions: Extracellular Nef can induce apoptosis and lymphocyte depletion in vivo. Appropriate antibody response can block these effects, but the apoptotic motifs in Nef are thought to be poorly immunogenic.
Figures

Similar articles
-
Extracellular Nef protein targets CD4+ T cells for apoptosis by interacting with CXCR4 surface receptors.J Virol. 2004 Mar;78(6):3099-109. doi: 10.1128/jvi.78.6.3099-3109.2004. J Virol. 2004. PMID: 14990729 Free PMC article.
-
Adult AIDS-like disease in a novel inducible human immunodeficiency virus type 1 Nef transgenic mouse model: CD4+ T-cell activation is Nef dependent and can occur in the absence of lymphophenia.J Virol. 2009 Nov;83(22):11830-46. doi: 10.1128/JVI.01466-09. Epub 2009 Sep 9. J Virol. 2009. PMID: 19740990 Free PMC article.
-
In vivo mutational analysis of the N-terminal region of HIV-1 Nef reveals critical motifs for the development of an AIDS-like disease in CD4C/HIV transgenic mice.Virology. 2004 Oct 1;327(2):273-86. doi: 10.1016/j.virol.2004.06.028. Virology. 2004. PMID: 15351215
-
Characterization of Nef-CXCR4 interactions important for apoptosis induction.J Virol. 2004 Oct;78(20):11084-96. doi: 10.1128/JVI.78.20.11084-11096.2004. J Virol. 2004. PMID: 15452229 Free PMC article.
-
Could Nef and Vpr proteins contribute to disease progression by promoting depletion of bystander cells and prolonged survival of HIV-infected cells?Biochem Biophys Res Commun. 2000 Jan 27;267(3):677-85. doi: 10.1006/bbrc.1999.1708. Biochem Biophys Res Commun. 2000. PMID: 10673351 Review.
Cited by
-
Mechanisms of HIV-associated lymphocyte apoptosis: 2010.Cell Death Dis. 2010 Nov 11;1(11):e99. doi: 10.1038/cddis.2010.77. Cell Death Dis. 2010. PMID: 21368875 Free PMC article. Review.
-
The interaction between human papillomavirus and other viruses.Virus Res. 2017 Mar 2;231:139-147. doi: 10.1016/j.virusres.2016.11.002. Epub 2016 Nov 5. Virus Res. 2017. PMID: 27826043 Free PMC article. Review.
-
Cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection.Nature. 2014 Jan 23;505(7484):509-14. doi: 10.1038/nature12940. Nature. 2014. PMID: 24356306 Free PMC article.
-
Secretion modification region-derived peptide blocks exosome release and mediates cell cycle arrest in breast cancer cells.Oncotarget. 2017 Feb 14;8(7):11302-11315. doi: 10.18632/oncotarget.14513. Oncotarget. 2017. PMID: 28076321 Free PMC article.
References
-
- Fujii Y, Otake K, Tashiro M, Adachi A. Soluble Nef antigen of HIV-1 is cytotoxic for human CD4+ T cells. FEBS Lett. 1996;393:93–96. - PubMed
-
- Guy B, Riviere Y, Dott K, Regnault A, Kieny MP. Mutational analysis of the HIV Nef protein. Virology. 1990;176:413–425. - PubMed
-
- Addo MM, Yu XG, Rathod A, et al. Comprehensive epitope analysis of human immunodeficiency virus type 1 (HIV-1)-specific T-cell responses directed against the entire expressed HIV-1 genome demonstrate broadly directed responses, but no correlation to viral load. J Virol. 2003;77:2081–2092. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
