Annexin-A1: a pivotal regulator of the innate and adaptive immune systems
- PMID: 18641677
- PMCID: PMC2538690
- DOI: 10.1038/bjp.2008.252
Annexin-A1: a pivotal regulator of the innate and adaptive immune systems
Abstract
The glucocorticoids are the most potent anti-inflammatory drugs that we possess and are effective in a wide variety of diseases. Although their action is known to involve receptor mediated changes in gene transcription, the exact mechanisms whereby these bring about their pleiotropic action in inflammation are yet to be totally understood. Whilst many different genes are regulated by the glucocorticoids, we have identified one particular protein-annexin A1 (Anx-A1)-whose synthesis and release is strongly regulated by the glucocorticoids in many cell types. The biology of this protein, as revealed by studies using transgenic animals, peptide mimetics and neutralizing antibodies, speaks to its role as a key modulator of both of the innate and adaptive immune systems. The mechanism whereby this protein exerts its effects is likely to be through the FPR receptor family-a hitherto rather enigmatic family of G protein coupled receptors, which are increasingly implicated in the regulation of many inflammatory processes. Here we review some of the key findings that have led up to the elucidation of this key pathway in inflammatory resolution.
Figures
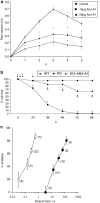
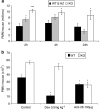

Similar articles
-
Annexin A1 and glucocorticoids as effectors of the resolution of inflammation.Nat Rev Immunol. 2009 Jan;9(1):62-70. doi: 10.1038/nri2470. Nat Rev Immunol. 2009. PMID: 19104500 Review.
-
[Annexin-1: 2nd messanger of the anti-inflammatory actions of glucocorticoids].Acta Reumatol Port. 2006 Oct-Dec;31(4):293-302. Acta Reumatol Port. 2006. PMID: 17334042 Review. Portuguese.
-
The role of the Annexin-A1/FPR2 system in the regulation of mast cell degranulation provoked by compound 48/80 and in the inhibitory action of nedocromil.Int Immunopharmacol. 2016 Mar;32:87-95. doi: 10.1016/j.intimp.2016.01.003. Epub 2016 Jan 21. Int Immunopharmacol. 2016. PMID: 26803520 Free PMC article.
-
Annexin A1: potential for glucocorticoid sparing in RA.Nat Rev Rheumatol. 2013 Oct;9(10):595-603. doi: 10.1038/nrrheum.2013.126. Epub 2013 Aug 20. Nat Rev Rheumatol. 2013. PMID: 23958797 Review.
-
On the adaptive nature of annexin-A1.Curr Opin Pharmacol. 2009 Aug;9(4):521-8. doi: 10.1016/j.coph.2009.04.007. Epub 2009 May 27. Curr Opin Pharmacol. 2009. PMID: 19481503 Review.
Cited by
-
Anti-allergic cromones inhibit histamine and eicosanoid release from activated human and murine mast cells by releasing Annexin A1.PLoS One. 2013;8(3):e58963. doi: 10.1371/journal.pone.0058963. Epub 2013 Mar 18. PLoS One. 2013. PMID: 23527056 Free PMC article.
-
Host biomarkers of invasive pulmonary aspergillosis to monitor therapeutic response.Antimicrob Agents Chemother. 2014 Jun;58(6):3373-8. doi: 10.1128/AAC.02482-14. Epub 2014 Mar 31. Antimicrob Agents Chemother. 2014. PMID: 24687510 Free PMC article.
-
Endothelial microparticles released by activated protein C protect beta cells through EPCR/PAR1 and annexin A1/FPR2 pathways in islets.J Cell Mol Med. 2017 Nov;21(11):2759-2772. doi: 10.1111/jcmm.13191. Epub 2017 May 19. J Cell Mol Med. 2017. PMID: 28524456 Free PMC article.
-
Resolution of Cochlear Inflammation: Novel Target for Preventing or Ameliorating Drug-, Noise- and Age-related Hearing Loss.Front Cell Neurosci. 2017 Jul 7;11:192. doi: 10.3389/fncel.2017.00192. eCollection 2017. Front Cell Neurosci. 2017. PMID: 28736517 Free PMC article. Review.
-
Divergent Annexin A1 expression in periphery and gut is associated with systemic immune activation and impaired gut immune response during SIV infection.Sci Rep. 2016 Aug 3;6:31157. doi: 10.1038/srep31157. Sci Rep. 2016. PMID: 27484833 Free PMC article.
References
-
- Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942–949. - PubMed
-
- Ahn SH, Sawada H, Ro JY, Nicolson GL. Differential expression of annexin I in human mammary ductal epithelial cells in normal and benign and malignant breast tissues. Clin Exp Metastasis. 1997;15:151–156. - PubMed
-
- Akama H, Tanaka H, Kawai S. Possible mechanisms of glucocorticoid—unresponsive pyrexia. Defect in lipocortin 1. Mater Med Pol. 1995;27:75–78. - PubMed
-
- Aksentijevich S, Whitfield HJ, Jr, Young WS, III, Wilder RL, Chrousos GP, Gold PW, et al. Arthritis-susceptible Lewis rats fail to emerge from the stress hyporesponsive period. Brain Res Dev Brain Res. 1992;65:115–118. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

