Increased expression of CCL2 in insulin-producing cells of transgenic mice promotes mobilization of myeloid cells from the bone marrow, marked insulitis, and diabetes
- PMID: 18633103
- PMCID: PMC2570399
- DOI: 10.2337/db08-0625
Increased expression of CCL2 in insulin-producing cells of transgenic mice promotes mobilization of myeloid cells from the bone marrow, marked insulitis, and diabetes
Abstract
Objective: To define the mechanisms underlying the accumulation of monocytes/macrophages in the islets of Langerhans.
Research design and methods: We tested the hypothesis that macrophage accumulation into the islets is caused by overexpression of the chemokine CCL2. To test this hypothesis, we generated transgenic mice and evaluated the cellular composition of the islets by immunohistochemistry and flow cytometry. We determined serum levels of CCL2 by enzyme-linked immunosorbent assay, determined numbers of circulating monocytes, and tested whether CCL2 could mobilize monocytes from the bone marrow directly. We examined development of diabetes over time and tested whether CCL2 effects could be eliminated by deletion of its receptor, CCR2.
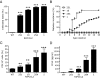
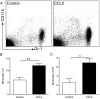
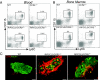
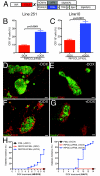
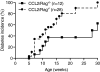
Results: Expression of CCL2 by beta-cells was associated with increased numbers of monocytes in circulation and accumulation of macrophages in the islets of transgenic mice. These changes were promoted by combined actions of CCL2 at the level of the bone marrow and the islets and were not seen in animals in which the CCL2 receptor (CCR2) was inactivated. Mice expressing higher levels of CCL2 in the islets developed diabetes spontaneously. The development of diabetes was correlated with the accumulation of large numbers of monocytes in the islets and did not depend on T- and B-cells. Diabetes could also be induced in normoglycemic mice expressing low levels of CCL2 by increasing the number of circulating myeloid cells.
Conclusions: These results indicate that CCL2 promotes monocyte recruitment by acting both locally and remotely and that expression of CCL2 by insulin-producing cells can lead to insulitis and islet destruction.
Figures
Comment in
-
Big mac attack: does it play a direct role for monocytes/macrophages in type 1 diabetes?Diabetes. 2008 Nov;57(11):2922-3. doi: 10.2337/db08-1007. Diabetes. 2008. PMID: 18971442 Free PMC article. No abstract available.
Similar articles
-
Pancreatic islet expression of chemokine CCL2 suppresses autoimmune diabetes via tolerogenic CD11c+ CD11b+ dendritic cells.Proc Natl Acad Sci U S A. 2012 Feb 28;109(9):3457-62. doi: 10.1073/pnas.1115308109. Epub 2012 Feb 10. Proc Natl Acad Sci U S A. 2012. PMID: 22328150 Free PMC article.
-
Acute skeletal muscle injury: CCL2 expression by both monocytes and injured muscle is required for repair.FASEB J. 2011 Oct;25(10):3344-55. doi: 10.1096/fj.10-178939. Epub 2011 Jun 22. FASEB J. 2011. PMID: 21697550 Free PMC article.
-
Modulating CCR2 and CCL2 at the blood-brain barrier: relevance for multiple sclerosis pathogenesis.Brain. 2006 Jan;129(Pt 1):212-23. doi: 10.1093/brain/awh655. Epub 2005 Oct 17. Brain. 2006. PMID: 16230319
-
CCR2-positive monocytes recruited to inflamed lungs downregulate local CCL2 chemokine levels.Am J Physiol Lung Cell Mol Physiol. 2005 Feb;288(2):L350-8. doi: 10.1152/ajplung.00061.2004. Epub 2004 Oct 29. Am J Physiol Lung Cell Mol Physiol. 2005. PMID: 15516494
-
Current advances and future prospects in production of recombinant insulin and other proteins to treat diabetes mellitus.Biotechnol Lett. 2022 Jun;44(5-6):643-669. doi: 10.1007/s10529-022-03247-w. Epub 2022 Apr 17. Biotechnol Lett. 2022. PMID: 35430708 Review.
Cited by
-
Islet inflammation: a unifying target for diabetes treatment?Trends Endocrinol Metab. 2013 Jul;24(7):351-60. doi: 10.1016/j.tem.2013.01.007. Epub 2013 Feb 26. Trends Endocrinol Metab. 2013. PMID: 23484621 Free PMC article. Review.
-
IL-17A increases the expression of proinflammatory chemokines in human pancreatic islets.Diabetologia. 2014 Mar;57(3):502-11. doi: 10.1007/s00125-013-3135-2. Epub 2013 Dec 19. Diabetologia. 2014. PMID: 24352375
-
Role of DNA-LL37 complexes in the activation of plasmacytoid dendritic cells and monocytes in subjects with type 1 diabetes.Sci Rep. 2020 Jun 1;10(1):8896. doi: 10.1038/s41598-020-65851-y. Sci Rep. 2020. PMID: 32483133 Free PMC article.
-
Pathogenic mechanisms in type 1 diabetes: the islet is both target and driver of disease.Rev Diabet Stud. 2012 Winter;9(4):148-68. doi: 10.1900/RDS.2012.9.148. Epub 2012 Dec 28. Rev Diabet Stud. 2012. PMID: 23804258 Free PMC article. Review.
-
Immune cell trafficking to the islets during type 1 diabetes.Clin Exp Immunol. 2019 Dec;198(3):314-325. doi: 10.1111/cei.13353. Epub 2019 Aug 30. Clin Exp Immunol. 2019. PMID: 31343073 Free PMC article. Review.
References
-
- Kolb H, Kolb-Bachofen V, Roep BO: Autoimmune versus inflammatory type I diabetes: a controversy? Immunol Today 16: 170–172, 1995 - PubMed
-
- Atkinson MA, Eisenbarth GS: Type 1 diabetes: new perspectives on disease pathogenesis and treatment. Lancet 358: 221–229, 2001 - PubMed
-
- Jansen A, Homo-Delarche F, Hooijkaas H, Leenen PJ, Dardenne M, Drexhage HA: Immunohistochemical characterization of monocytes-macrophages and dendritic cells involved in the initiation of the insulitis and β-cell destruction in NOD mice. Diabetes 43: 667–675, 1994 - PubMed
-
- Rosmalen JG, Martin T, Dobbs C, Voerman JS, Drexhage HA, Haskins K, Leenen PJ: Subsets of macrophages and dendritic cells in nonobese diabetic mouse pancreatic inflammatory infiltrates: correlation with the development of diabetes. Lab Invest 80: 23–30, 2000 - PubMed
-
- Nikolic T, Geutskens SB, van Rooijen N, Drexhage HA, Leenen PJ: Dendritic cells and macrophages are essential for the retention of lymphocytes in (peri)-insulitis of the nonobese diabetic mouse: a phagocyte depletion study. Lab Invest 85: 487–501, 2005 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

