Down-regulation of neurocan expression in reactive astrocytes promotes axonal regeneration and facilitates the neurorestorative effects of bone marrow stromal cells in the ischemic rat brain
- PMID: 18618668
- PMCID: PMC2575136
- DOI: 10.1002/glia.20722
Down-regulation of neurocan expression in reactive astrocytes promotes axonal regeneration and facilitates the neurorestorative effects of bone marrow stromal cells in the ischemic rat brain
Abstract
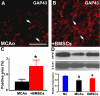
The glial scar, a primarily astrocytic structure bordering the infarct tissue inhibits axonal regeneration after stroke. Neurocan, an axonal extension inhibitory molecule, is up-regulated in the scar region after stroke. Bone marrow stromal cells (BMSCs) reduce the thickness of glial scar wall and facilitate axonal remodeling in the ischemic boundary zone. To further clarify the role of BMSCs in axonal regeneration and its underlying mechanism, the current study focused on the effect of BMSCs on neurocan expression in the ischemic brain. Thirty-one adult male Wistar rats were subjected to 2 h of middle cerebral artery occlusion followed by an injection of 3 x 10(6) rat BMSCs (n = 16) or phosphate-buffered saline (n = 15) into the tail vein 24 h later. Animals were sacrificed at 8 days after stroke. Immunostaining analysis showed that reactive astrocytes were the primary source of neurocan, and BMSC-treated animals had significantly lower neurocan and higher growth associated protein 43 expression in the penumbral region compared with control rats, which was confirmed by Western blot analysis of the brain tissue. To further investigate the effects of BMSCs on astrocyte neurocan expression, single reactive astrocytes were collected from the ischemic boundary zone using laser capture microdissection. Neurocan gene expression was significantly down-regulated in rats receiving BMSC transplantation (n = 4/group). Primary cultured astrocytes showed similar alterations; BMSC coculture during reoxygenation abolished the up-regulation of neurocan gene in astrocytes undergoing oxygen-glucose deprivation (n = 3/group). Our data suggest that BMSCs promote axonal regeneration by reducing neurocan expression in peri-infarct astrocytes.
Copyright 2008 Wiley-Liss, Inc.
Figures


Similar articles
-
Synergistic effects of repeated transcranial magnetic stimulation and mesenchymal stem cells transplantation on alleviating neuroinflammation and PANoptosis in cerebral ischemia.J Neuroinflammation. 2024 Nov 30;21(1):311. doi: 10.1186/s12974-024-03302-5. J Neuroinflammation. 2024. PMID: 39616364 Free PMC article.
-
Comparison of Two Modern Survival Prediction Tools, SORG-MLA and METSSS, in Patients With Symptomatic Long-bone Metastases Who Underwent Local Treatment With Surgery Followed by Radiotherapy and With Radiotherapy Alone.Clin Orthop Relat Res. 2024 Dec 1;482(12):2193-2208. doi: 10.1097/CORR.0000000000003185. Epub 2024 Jul 23. Clin Orthop Relat Res. 2024. PMID: 39051924
-
Neurotrophic and growth factor gene expression profiling of mouse bone marrow stromal cells induced by ischemic brain extracts.Neuropathology. 2007 Aug;27(4):355-63. doi: 10.1111/j.1440-1789.2007.00792.x. Neuropathology. 2007. PMID: 17899689 Free PMC article.
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
Trends in Surgical and Nonsurgical Aesthetic Procedures: A 14-Year Analysis of the International Society of Aesthetic Plastic Surgery-ISAPS.Aesthetic Plast Surg. 2024 Oct;48(20):4217-4227. doi: 10.1007/s00266-024-04260-2. Epub 2024 Aug 5. Aesthetic Plast Surg. 2024. PMID: 39103642 Review.
Cited by
-
Delayed Chronic Acidic Postconditioning Improves Poststroke Motor Functional Recovery and Brain Tissue Repair by Activating Proton-Sensing TDAG8.Transl Stroke Res. 2024 Jun;15(3):620-635. doi: 10.1007/s12975-023-01143-7. Epub 2023 Feb 28. Transl Stroke Res. 2024. PMID: 36853417
-
Progress in Mesenchymal Stem Cell Therapy for Ischemic Stroke.Stem Cells Int. 2021 Jun 15;2021:9923566. doi: 10.1155/2021/9923566. eCollection 2021. Stem Cells Int. 2021. PMID: 34221026 Free PMC article. Review.
-
Neuroprotection in preterm infants.Biomed Res Int. 2015;2015:257139. doi: 10.1155/2015/257139. Epub 2015 Jan 11. Biomed Res Int. 2015. PMID: 25650134 Free PMC article. Review.
-
Oxygen-glucose deprivation induced glial scar-like change in astrocytes.PLoS One. 2012;7(5):e37574. doi: 10.1371/journal.pone.0037574. Epub 2012 May 22. PLoS One. 2012. PMID: 22629422 Free PMC article.
-
Increasing tPA activity in astrocytes induced by multipotent mesenchymal stromal cells facilitate neurite outgrowth after stroke in the mouse.PLoS One. 2010 Feb 3;5(2):e9027. doi: 10.1371/journal.pone.0009027. PLoS One. 2010. PMID: 20140248 Free PMC article.
References
-
- Bovolenta P, Wandosell F, Nieto-Sampedro M. CNS glial scar tissue: a source of molecules which inhibit central neurite outgrowth. Prog Brain Res. 1992;94:367–79. - PubMed
-
- Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98(5):1076–84. - PubMed
-
- Chen H, Chopp M, Zhang ZG, Garcia JH. The effect of hypothermia on transient middle cerebral artery occlusion in the rat. J Cereb Blood Flow Metab. 1992;12(4):621–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

