A comprehensive analysis of the dual roles of BMPs in regulating adipogenic and osteogenic differentiation of mesenchymal progenitor cells
- PMID: 18616389
- PMCID: PMC3132950
- DOI: 10.1089/scd.2008.0130
A comprehensive analysis of the dual roles of BMPs in regulating adipogenic and osteogenic differentiation of mesenchymal progenitor cells
Abstract
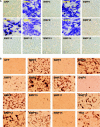
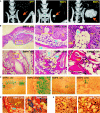
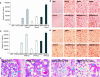
Pluripotent mesenchymal stem cells (MSCs) are bone marrow stromal progenitor cells that can differentiate into osteogenic, chondrogenic, adipogenic, and myogenic lineages. Several signaling pathways have been shown to regulate the lineage commitment and terminal differentiation of MSCs. Here, we conducted a comprehensive analysis of the 14 types of bone morphogenetic protein (BMPs) for their abilities to regulate multilineage specific differentiation of MSCs. We found that most BMPs exhibited distinct abilities to regulate the expression of Runx2, Sox9, MyoD, and PPARgamma2. Further analysis indicated that BMP-2, BMP-4, BMP-6, BMP-7, and BMP-9 effectively induced both adipogenic and osteogenic differentiation in vitro and in vivo. BMP-induced commitment to osteogenic or adipogenic lineage was shown to be mutually exclusive. Overexpression of Runx2 enhanced BMP-induced osteogenic differentiation, whereas knockdown of Runx2 expression diminished BMP-induced bone formation with a decrease in adipocyte accumulation in vivo. Interestingly, overexpression of PPARgamma2 not only promoted adipogenic differentiation, but also enhanced osteogenic differentiation upon BMP-2, BMP-6, and BMP-9 stimulation. Conversely, MSCs with PPARgamma2 knockdown or mouse embryonic fibroblasts derived from PPARgamma2(-/-) mice exhibited a marked decrease in adipogenic differentiation, coupled with reduced osteogenic differentiation and diminished mineralization upon BMP-9 stimulation, suggesting that PPARgamma2 may play a role in BMP-induced osteogenic and adipogenic differentiation. Thus, it is important to understand the molecular mechanism behind BMP-regulated lineage divergence during MSC differentiation, as this knowledge could help us to understand the pathogenesis of skeletal diseases and may lead to the development of strategies for regenerative medicine.
Figures
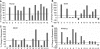


Similar articles
-
Hey1 basic helix-loop-helix protein plays an important role in mediating BMP9-induced osteogenic differentiation of mesenchymal progenitor cells.J Biol Chem. 2009 Jan 2;284(1):649-659. doi: 10.1074/jbc.M806389200. Epub 2008 Nov 5. J Biol Chem. 2009. PMID: 18986983 Free PMC article.
-
A novel PPARγ2 modulator sLZIP controls the balance between adipogenesis and osteogenesis during mesenchymal stem cell differentiation.Cell Death Differ. 2014 Oct;21(10):1642-55. doi: 10.1038/cdd.2014.80. Epub 2014 Jun 20. Cell Death Differ. 2014. PMID: 24948012 Free PMC article.
-
The role of BMP-7 in chondrogenic and osteogenic differentiation of human bone marrow multipotent mesenchymal stromal cells in vitro.J Cell Biochem. 2010 Feb 1;109(2):406-16. doi: 10.1002/jcb.22412. J Cell Biochem. 2010. PMID: 19950204
-
PPARγ and Wnt Signaling in Adipogenic and Osteogenic Differentiation of Mesenchymal Stem Cells.Curr Stem Cell Res Ther. 2016;11(3):216-25. doi: 10.2174/1574888x10666150519093429. Curr Stem Cell Res Ther. 2016. PMID: 25986621 Review.
-
The role of microRNAs in cell fate determination of mesenchymal stem cells: balancing adipogenesis and osteogenesis.BMB Rep. 2015 Jun;48(6):319-23. doi: 10.5483/bmbrep.2015.48.6.206. BMB Rep. 2015. PMID: 25341923 Free PMC article. Review.
Cited by
-
Decellularized liver scaffolds effectively support the proliferation and differentiation of mouse fetal hepatic progenitors.J Biomed Mater Res A. 2014 Apr;102(4):1017-25. doi: 10.1002/jbm.a.34764. Epub 2013 Jun 4. J Biomed Mater Res A. 2014. PMID: 23625886 Free PMC article.
-
Hydrogel-based delivery system applied in the local anti-osteoporotic bone defects.Front Bioeng Biotechnol. 2022 Nov 11;10:1058300. doi: 10.3389/fbioe.2022.1058300. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36440439 Free PMC article. Review.
-
C1q/tumor necrosis factor-related protein 11 (CTRP11), a novel adipose stroma-derived regulator of adipogenesis.J Biol Chem. 2013 Apr 12;288(15):10214-29. doi: 10.1074/jbc.M113.458711. Epub 2013 Feb 28. J Biol Chem. 2013. PMID: 23449976 Free PMC article.
-
Enhanced bone formation of calvarial bone defects by low-intensity pulsed ultrasound and recombinant human bone morphogenetic protein-9: a preliminary experimental study in rats.Clin Oral Investig. 2021 Oct;25(10):5917-5927. doi: 10.1007/s00784-021-03897-6. Epub 2021 Mar 23. Clin Oral Investig. 2021. PMID: 33755786
-
Long-Term Changes in Adipose Tissue in the Newly Formed Bone Induced by Recombinant Human BMP-2 In Vivo.Biomimetics (Basel). 2023 Jan 13;8(1):33. doi: 10.3390/biomimetics8010033. Biomimetics (Basel). 2023. PMID: 36648819 Free PMC article.
References
-
- Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. - PubMed
-
- Aubin JE. Bone stem cells. J Cell Biochem Suppl. 1998;31:73–82. - PubMed
-
- Pittenger MF. Mackay AM. Beck SC. Jaiswal RK. Douglas R. Mosca JD. Moorman MA. Simonetti DW. Craig S. Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. - PubMed
-
- Ducy P. Schinke T. Karsenty G. The osteoblast: a sophisticated fibroblast under central surveillance. Science. 2000;289:1501–1504. - PubMed
-
- Caplan AI. Bruder SP. Mesenchymal stem cells: building blocks for molecular medicine in the 21st century. Trends in Mol Med. 2001;7:259–264. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

