Behavioral states, network states, and sensory response variability
- PMID: 18614753
- PMCID: PMC2544460
- DOI: 10.1152/jn.90592.2008
Behavioral states, network states, and sensory response variability
Abstract
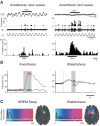
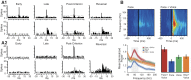
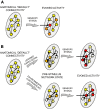
We review data demonstrating that single-neuron sensory responses change with the states of the neural networks (indexed in terms of spectral properties of local field potentials) in which those neurons are embedded. We start with broad network changes--different levels of anesthesia and sleep--and then move to studies demonstrating that the sensory response plasticity associated with attention and experience can also be conceptualized as functions of network state changes. This leads naturally to the recent data that can be interpreted to suggest that even brief experience can change sensory responses via changes in network states and that trial-to-trial variability in sensory responses is a nonrandom function of network fluctuations, as well. We suggest that the CNS may have evolved specifically to deal with stimulus variability and that the coupling with network states may be central to sensory processing.
Figures


Similar articles
-
From sensors to spikes: evolving receptive fields to enhance sensorimotor information in a robot-arm.Int J Neural Syst. 2012 Aug;22(4):1250013. doi: 10.1142/S012906571250013X. Epub 2012 Jul 12. Int J Neural Syst. 2012. PMID: 22830963
-
Measuring direction in the coupling of biological oscillators: a case study for electroreceptors of paddlefish.Chaos. 2006 Jun;16(2):026111. doi: 10.1063/1.2201466. Chaos. 2006. PMID: 16822043
-
Correlations between the activity of sensory neurons and behavior: how much do they tell us about a neuron's causality?Curr Opin Neurobiol. 2010 Jun;20(3):376-81. doi: 10.1016/j.conb.2010.05.002. Curr Opin Neurobiol. 2010. PMID: 20545019 Free PMC article. Review.
-
Neural processing as causal inference.Curr Opin Neurobiol. 2011 Oct;21(5):774-81. doi: 10.1016/j.conb.2011.05.018. Curr Opin Neurobiol. 2011. PMID: 21742484 Review.
-
Interactions between intrinsic membrane and emerging network properties determine signal processing in central vestibular neurons.Exp Brain Res. 2011 May;210(3-4):437-49. doi: 10.1007/s00221-011-2585-3. Epub 2011 Mar 4. Exp Brain Res. 2011. PMID: 21374082 Review.
Cited by
-
Visually cued action timing in the primary visual cortex.Neuron. 2015 Apr 8;86(1):319-30. doi: 10.1016/j.neuron.2015.02.043. Epub 2015 Mar 26. Neuron. 2015. PMID: 25819611 Free PMC article.
-
Dynamics of multistable states during ongoing and evoked cortical activity.J Neurosci. 2015 May 27;35(21):8214-31. doi: 10.1523/JNEUROSCI.4819-14.2015. J Neurosci. 2015. PMID: 26019337 Free PMC article.
-
Removal of default state-associated inhibition during repetition priming improves response articulation.J Neurosci. 2012 Dec 5;32(49):17740-52. doi: 10.1523/JNEUROSCI.4137-12.2012. J Neurosci. 2012. PMID: 23223294 Free PMC article.
-
Reciprocal relationships between sleep and smell.Front Neural Circuits. 2022 Dec 22;16:1076354. doi: 10.3389/fncir.2022.1076354. eCollection 2022. Front Neural Circuits. 2022. PMID: 36619661 Free PMC article. Review.
-
Stimulus presentation can enhance spiking irregularity across subcortical and cortical regions.PLoS Comput Biol. 2022 Jul 5;18(7):e1010256. doi: 10.1371/journal.pcbi.1010256. eCollection 2022 Jul. PLoS Comput Biol. 2022. PMID: 35789328 Free PMC article.
References
-
- Adrian ED The electrical activity of the mammalian olfactory bulb. Electroencephalogr Clin Neurophysiol 2: 377–388, 1950. - PubMed
-
- Arcediano F, Miller RR. Some constraints for models of timing: a temporal coding hypothesis perspective. Learn Motiv 33: 105–123, 2002.
-
- Arieli A, Sterkin A, Grinvald A, Aertsen A. Dynamics of ongoing activity: explanation of the large variability in evoked cortical responses. Science 273: 1868–1871, 1996. - PubMed
-
- Bahrick LE, Lickliter R, Flom R. Intersensory redundancy guides the development of selective attention, perception, and cognition in infancy. Curr Direct Psychol Sci 13: 99–102, 2004.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

