Production of a specific extracellular inhibitor of TRPM3 channels
- PMID: 18604232
- PMCID: PMC2579657
- DOI: 10.1038/bjp.2008.283
Production of a specific extracellular inhibitor of TRPM3 channels
Abstract
Background and purpose: Isoform-specific ion channel blockers are useful for target validation in drug discovery and can provide the basis for new therapeutic agents and aid in determination of physiological functions of ion channels. The aim of this study was to generate a specific blocker of human TRPM3 channels as a tool to help investigations of this member of the TRP cationic channel family.
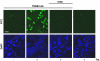
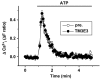
Experimental approach: A polyclonal antibody (TM3E3) was made to a conserved peptide of the third extracellular (E3) loop of TRPM3 and tested for binding and functional effect. Studies of channel activity were made by whole-cell planar patch-clamp and fura-2 intracellular Ca(2+) measurement.
Key results: Ionic current mediated by TRPM3 was inhibited partially by TM3E3 over a period of 5-10 min. Ca(2+) entry in TRPM3-expressing cells was also partially inhibited by TM3E3 in a peptide-specific manner and independently of the type of agonist used to activate TRPM3. TM3E3 had no effect on TRPC5, TRPV4, TRPM2 or an endogenous ATP response.
Conclusions and implications: The data show the successful development of a specific TRPM3 inhibitor and give further confidence in E3 targeting as an approach to producing isoform-specific ion channel blockers.
Figures

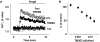
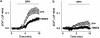

Similar articles
-
Inhibition of endothelial cell Ca²⁺ entry and transient receptor potential channels by Sigma-1 receptor ligands.Br J Pharmacol. 2013 Mar;168(6):1445-55. doi: 10.1111/bph.12041. Br J Pharmacol. 2013. PMID: 23121507 Free PMC article.
-
Rapid and contrasting effects of rosiglitazone on transient receptor potential TRPM3 and TRPC5 channels.Mol Pharmacol. 2011 Jun;79(6):1023-30. doi: 10.1124/mol.110.069922. Epub 2011 Mar 15. Mol Pharmacol. 2011. PMID: 21406603 Free PMC article.
-
Activation of the melastatin-related cation channel TRPM3 by D-erythro-sphingosine [corrected].Mol Pharmacol. 2005 Mar;67(3):798-805. doi: 10.1124/mol.104.006734. Epub 2004 Nov 18. Mol Pharmacol. 2005. PMID: 15550678
-
TRPM3, a biophysical enigma?Biochem Soc Trans. 2007 Feb;35(Pt 1):89-90. doi: 10.1042/BST0350089. Biochem Soc Trans. 2007. PMID: 17233609 Review.
-
Transient receptor potential TRPM3 channels: Pharmacology, signaling, and biological functions.Pharmacol Res. 2017 Oct;124:92-99. doi: 10.1016/j.phrs.2017.07.014. Epub 2017 Jul 16. Pharmacol Res. 2017. PMID: 28720517 Review.
Cited by
-
Antibodies to the extracellular pore loop of TRPM8 act as antagonists of channel activation.PLoS One. 2014 Sep 9;9(9):e107151. doi: 10.1371/journal.pone.0107151. eCollection 2014. PLoS One. 2014. PMID: 25203266 Free PMC article.
-
Activation and inhibition of transient receptor potential TRPM3-induced gene transcription.Br J Pharmacol. 2014 May;171(10):2645-58. doi: 10.1111/bph.12524. Br J Pharmacol. 2014. PMID: 24895737 Free PMC article.
-
CREB, AP-1, ternary complex factors and MAP kinases connect transient receptor potential melastatin-3 (TRPM3) channel stimulation with increased c-Fos expression.Br J Pharmacol. 2016 Jan;173(2):305-18. doi: 10.1111/bph.13372. Epub 2015 Dec 19. Br J Pharmacol. 2016. PMID: 26493679 Free PMC article.
-
Primidone inhibits TRPM3 and attenuates thermal nociception in vivo.Pain. 2017 May;158(5):856-867. doi: 10.1097/j.pain.0000000000000846. Pain. 2017. PMID: 28106668 Free PMC article.
-
Inhibition of endothelial cell Ca²⁺ entry and transient receptor potential channels by Sigma-1 receptor ligands.Br J Pharmacol. 2013 Mar;168(6):1445-55. doi: 10.1111/bph.12041. Br J Pharmacol. 2013. PMID: 23121507 Free PMC article.
References
-
- Chioni AM, Fraser SP, Pani F, Foran P, Wilkin GP, Diss JK, et al. A novel polyclonal antibody specific for the NaV1.5 voltage-gated Na+ channel ‘neonatal' splice form. J Neurosci Methods. 2005;147:88–98. - PubMed
-
- Gomez-Varela D, Zwick-Wallasch E, Knotgen H, Sanchez A, Hettmann T, Ossipov D, et al. Monoclonal antibody blockade of the human Eag1 potassium channel function exerts antitumor activity. Cancer Res. 2007;67:7343–7349. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

