Connexin40 imparts conduction heterogeneity to atrial tissue
- PMID: 18599871
- PMCID: PMC2925175
- DOI: 10.1161/CIRCRESAHA.107.168997
Connexin40 imparts conduction heterogeneity to atrial tissue
Abstract
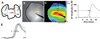
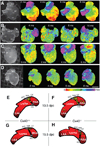
Impulse propagation in cardiac tissue is a complex process in which intercellular coupling through gap junction channels is a critical component. Connexin40 (Cx40) is an abundant gap junction protein that is expressed in atrial myocytes. Alterations in the expression of Cx40 have been implicated in atrial arrhythmogenesis. The purpose of the current study was to assess the role of Cx40 in atrial impulse propagation. High-resolution optical mapping was used to study conduction in the right and left atrial appendages of isolated Langendorff-perfused murine hearts. Wild-type (Cx40(+/+)), heterozygous (Cx40(+/-)), and knockout (Cx40(-/-)) mice, both adult and embryonic, were studied to assess the effects of reduced Cx40 expression on sinus node function and conduction velocity at different pacing cycle lengths (100 and 60 ms). In both adult and late-stage embryonic Cx40(+/+) mice, heterogeneity in CV was found between the right and left atrial appendages. Either partial (Cx40(+/-)) or complete (Cx40(-/-)) deletion of Cx40 was associated with the loss of conduction heterogeneity in both adult and embryonic mice. Additionally, sinus node impulse initiation was found to be ectopic in Cx40(-/-) mice at 15.5 days postcoitus, whereas Cx40(+/+) mice showed normal activation occurring near the crista terminalis. Our findings suggest that Cx40 plays an essential role in establishing interatrial conduction velocity heterogeneity in the murine model. Additionally, we describe for the first time a developmental requirement for Cx40 in normal sinus node impulse initiation at 15.5 days postcoitus.
Figures



Similar articles
-
The role of connexin40 in developing atrial conduction.FEBS Lett. 2014 Apr 17;588(8):1465-9. doi: 10.1016/j.febslet.2014.01.032. Epub 2014 Jan 31. FEBS Lett. 2014. PMID: 24486905
-
Altered right atrial excitation and propagation in connexin40 knockout mice.Circulation. 2005 Oct 11;112(15):2245-53. doi: 10.1161/CIRCULATIONAHA.104.527325. Epub 2005 Oct 3. Circulation. 2005. PMID: 16203917 Free PMC article.
-
High-resolution optical mapping of the right bundle branch in connexin40 knockout mice reveals slow conduction in the specialized conduction system.Circ Res. 2000 Nov 10;87(10):929-36. doi: 10.1161/01.res.87.10.929. Circ Res. 2000. PMID: 11073890
-
[Cardiac arrhythmias in targeted connexin deficient mice: significance for the arrhythmia field].Z Kardiol. 2000 Dec;89(12):1108-18. doi: 10.1007/s003920070138. Z Kardiol. 2000. PMID: 11201026 Review. German.
-
Cardiac connexins and impulse propagation.J Mol Cell Cardiol. 2010 Jan;48(1):76-82. doi: 10.1016/j.yjmcc.2009.08.018. Epub 2009 Aug 31. J Mol Cell Cardiol. 2010. PMID: 19729017 Review.
Cited by
-
Permanent and Transient Electrophysiological Effects During Cardiac Cryoablation Documented by Optical Activation Mapping and Thermal Imaging.IEEE Trans Biomed Eng. 2018 Nov 9:10.1109/TBME.2018.2880408. doi: 10.1109/TBME.2018.2880408. Online ahead of print. IEEE Trans Biomed Eng. 2018. PMID: 30418875 Free PMC article.
-
Guide Cells Support Muscle Regeneration and Affect Neuro-Muscular Junction Organization.Int J Mol Sci. 2021 Feb 16;22(4):1939. doi: 10.3390/ijms22041939. Int J Mol Sci. 2021. PMID: 33669272 Free PMC article.
-
Atrial Fibrillation and Fibrosis: Beyond the Cardiomyocyte Centric View.Biomed Res Int. 2015;2015:798768. doi: 10.1155/2015/798768. Epub 2015 Jul 1. Biomed Res Int. 2015. PMID: 26229964 Free PMC article. Review.
-
Inherited and Acquired Rhythm Disturbances in Sick Sinus Syndrome, Brugada Syndrome, and Atrial Fibrillation: Lessons from Preclinical Modeling.Cells. 2021 Nov 15;10(11):3175. doi: 10.3390/cells10113175. Cells. 2021. PMID: 34831398 Free PMC article. Review.
-
Gap junction remodeling and spironolactone-dependent reverse remodeling in the hypertrophied heart.Circ Res. 2009 Feb 13;104(3):365-71. doi: 10.1161/CIRCRESAHA.108.184044. Epub 2008 Dec 18. Circ Res. 2009. PMID: 19096029 Free PMC article.
References
-
- Kleber AG, Fast VG, Rohr S. Continuous and discontinuous propagation. In: Zipes DP, Jalife J, editors. Cardiac Electrophysiology: From Cell to Bedside. Philadelphia: WB Saunders Co; 2000. pp. 205–213.
-
- Moreno AP. Biophysical properties of homomeric and heteromultimeric channels formed by cardiac connexins. Cardiovasc Res. 2004;62:276–286. - PubMed
-
- Saffitz JE, Lerner DL, Yamada KA. Gap junction distribution and regulation in the heart. In: Zipes DP, Jalife J, editors. Cardiac Electrophysiology: From Cell to Bedside. Vol IV. Philadelphia: WB Saunders; 2004. pp. 181–191.
-
- Jalife J, Morley GE, Vaidya D. Connexins and impulse propagation in the mouse heart. J Cardiovasc Electrophysiol. 1999;10:1649–1663. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

