Potential for modulation of the hydrophobic effect inside chaperonins
- PMID: 18599630
- PMCID: PMC2547425
- DOI: 10.1529/biophysj.108.131037
Potential for modulation of the hydrophobic effect inside chaperonins
Abstract
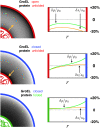
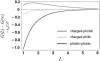
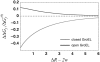
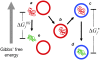
Despite the spontaneity of some in vitro protein-folding reactions, native folding in vivo often requires the participation of barrel-shaped multimeric complexes known as chaperonins. Although it has long been known that chaperonin substrates fold upon sequestration inside the chaperonin barrel, the precise mechanism by which confinement within this space facilitates folding remains unknown. We examine the possibility that the chaperonin mediates a favorable reorganization of the solvent for the folding reaction. We discuss the effect of electrostatic charge on solvent-mediated hydrophobic forces in an aqueous environment. Based on these physical arguments, we construct a simple, phenomenological theory for the thermodynamics of density and hydrogen-bond order fluctuations in liquid water. Within the framework of this model, we investigate the effect of confinement inside a chaperonin-like cavity on the configurational free energy of water by calculating solvent free energies for cavities corresponding to the different conformational states in the ATP-driven catalytic cycle of the prokaryotic chaperonin GroEL. Our findings suggest that one function of chaperonins may involve trapping unfolded proteins and subsequently exposing them to a microenvironment in which the hydrophobic effect, a crucial thermodynamic driving force for folding, is enhanced.
Figures
Similar articles
-
Exploring the kinetic requirements for enhancement of protein folding rates in the GroEL cavity.J Mol Biol. 1999 Apr 2;287(3):627-44. doi: 10.1006/jmbi.1999.2591. J Mol Biol. 1999. PMID: 10092464
-
Dual function of protein confinement in chaperonin-assisted protein folding.Cell. 2001 Oct 19;107(2):223-33. doi: 10.1016/s0092-8674(01)00517-7. Cell. 2001. PMID: 11672529
-
Effects of confinement in chaperonin assisted protein folding: rate enhancement by decreasing the roughness of the folding energy landscape.J Mol Biol. 2003 Sep 19;332(3):701-13. doi: 10.1016/s0022-2836(03)00929-x. J Mol Biol. 2003. PMID: 12963377
-
Reaction Cycle of Chaperonin GroEL via Symmetric "Football" Intermediate.J Mol Biol. 2015 Sep 11;427(18):2912-8. doi: 10.1016/j.jmb.2015.04.007. Epub 2015 Apr 18. J Mol Biol. 2015. PMID: 25900372 Review.
-
Two families of chaperonin: physiology and mechanism.Annu Rev Cell Dev Biol. 2007;23:115-45. doi: 10.1146/annurev.cellbio.23.090506.123555. Annu Rev Cell Dev Biol. 2007. PMID: 17489689 Review.
Cited by
-
GroEL/ES chaperonin modulates the mechanism and accelerates the rate of TIM-barrel domain folding.Cell. 2014 May 8;157(4):922-934. doi: 10.1016/j.cell.2014.03.038. Cell. 2014. PMID: 24813614 Free PMC article.
-
Reconciling theories of chaperonin accelerated folding with experimental evidence.Cell Mol Life Sci. 2010 Jan;67(2):255-76. doi: 10.1007/s00018-009-0164-6. Epub 2009 Oct 23. Cell Mol Life Sci. 2010. PMID: 19851829 Free PMC article. Review.
-
Derivation and assessment of phase-shifted, disordered vector field models for frustrated solvent interactions.J Chem Phys. 2013 Feb 28;138(8):085103. doi: 10.1063/1.4792638. J Chem Phys. 2013. PMID: 23464179 Free PMC article.
-
Slowdown of Water Dynamics from the Top to the Bottom of the GroEL Cavity.J Phys Chem Lett. 2021 Jun 24;12(24):5723-5730. doi: 10.1021/acs.jpclett.1c01216. Epub 2021 Jun 15. J Phys Chem Lett. 2021. PMID: 34129341 Free PMC article.
-
Action of the chaperonin GroEL/ES on a non-native substrate observed with single-molecule FRET.J Mol Biol. 2010 Aug 27;401(4):553-63. doi: 10.1016/j.jmb.2010.06.050. Epub 2010 Jun 30. J Mol Biol. 2010. PMID: 20600107 Free PMC article.
References
-
- Frydman, J. 2001. Folding of newly translated proteins in vivo: the role of molecular chaperones. Annu. Rev. Biochem. 70:603–647. - PubMed
-
- Hartl, F. U., and M. Hayer-Hartl. 2002. Molecular chaperones in the cytosol: from nascent chain to folded protein. Science. 295:1852–1858. - PubMed
-
- Sigler, P. B., Z. Xu, H. S. Rye, S. G. Burston, W. Fenton, and A. L. Horwich. 1998. Structure and function in GroEL-mediated protein folding. Annu. Rev. Biochem. 67:581–608. - PubMed
-
- Fenton, W. A., and A. L. Horwich. 2003. Chaperonin-mediated protein folding: fate of substrate polypeptide. Q. Rev. Biophys. 36:229–256. - PubMed
-
- Brinker, A., G. Pfeifer, M. J. Kerner, D. J. Naylor, F. U. Hartl, and M. Hayer-Hartl. 2001. Dual function of protein confinement in chaperonin-assisted protein folding. Cell. 107:223–233. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

